

UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a) of
the Securities Exchange Act of 1934
Filed by the Registrant ☒
Filed by a Party other than the Registrant ☐
Check the appropriate box:
| ☐ | Preliminary Proxy Statement | ☐ | Confidential, For Use of the Commission Only (as permitted by Rule14a-6(e)(2)) | |||
| ☒ | Definitive Proxy Statement | |||||
| ☐ | Definitive Additional Materials | |||||
| ☐ | Soliciting Material under§240.14a-12 |
TEVA PHARMACEUTICAL INDUSTRIES LIMITED
(Name of Registrant as Specified In Its Charter)
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
| ☒ | No fee required. | |||
| ☐ | Fee computed on table below per Exchange Act Rules14a-6(i) (1) and0-11. | |||
| (1) | Title of each class of securities to which transaction applies: | |||
| ||||
| (2) | Aggregate number of securities to which transaction applies: | |||
| ||||
| (3) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): | |||
| ||||
| (4) | Proposed maximum aggregate value of transaction: | |||
| ||||
| (5) | Total fee paid: | |||
| ||||
| ☐ | Fee paid previously with preliminary materials. | |||
| ☐ | Check box if any part of the fee is offset as provided by Exchange Act Rule0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. | |||
| (1) | Amount Previously Paid: | |||
| ||||
| (2) | Form, Schedule or Registration Statement No.: | |||
| ||||
| (3) | Filing Party: | |||
| ||||
| (4) | Date Filed: | |||
| ||||


|
| ||||
|
| |||
|

Notice of 20182021 Annual Meeting of Shareholders
| DATE AND TIME: | ||
| ||
| ITEMS OF BUSINESS: | Proposal 1: To appoint the following persons to
Proposal 2: To approve, on anon-binding advisory basis, the compensation for Teva’s named executive officers.
Proposal 3
In addition, shareholders will consider Teva’s annual consolidated financial statements for the year ended December 31,
The Board of Directors recommends that you vote FOR
Teva urges all of its shareholders to review its annual report (“Annual | |
| ITEMS OF BUSINESS: | Proposal 1: To appoint the following persons to Teva’s Board of Directors: Rosemary A. Crane, Abbas Hussain, Gerald M. Lieberman and Prof. Ronit Satchi-Fainaro to serve until our 2024 annual meeting of shareholders. Proposal 2: To approve, on Proposal 3: To appoint Kesselman & Kesselman, a member of PricewaterhouseCoopers International Ltd., as Teva’s independent registered public accounting firm until Teva’s 2022 annual meeting of shareholders. In addition, shareholders will consider Teva’s annual consolidated financial statements for the year ended December 31, | |
By Order of the Board of Directors,

Dov Bergwerk
Senior Vice President,
|
Important Notice Regarding the Availability of Proxy Materials for the Shareholder Meeting to be Held on June 5, 2018
The accompanying Proxy Statement and our Annual Report are available at www.tevapharm.com/2018proxymaterials. We expect the proxy materials to be mailed and/or made available on or before April 25, 2018.
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement i
Questions and Answers About the Annual Meeting
The Meeting
When and where will the Annual Meeting be held?
The 2018 Annual Meeting of Shareholders (the “Meeting” or the “its shareholders to review its annual report (“Annual Meeting”) of Teva Pharmaceutical Industries Limited (“we,” “us,” “our,” “Teva” or the“TevaCompany”) will be held atconducted in a virtual format.
Proposal 1: To appoint the following persons to Teva’s Board of Directors: Rosemary A. Crane, Abbas Hussain, Gerald M. Lieberman and Prof. Ronit Satchi-Fainaro to serve until our 2024 annual meeting of shareholders.
Proposal 2: To approve, on a non-binding advisory basis, the compensation for Teva’s named executive offices at 5 Basel Street, Petach Tikva, 4951033 Israel, on Tuesday, June 5, 2018, at 4:30 p.m.officers.
Proposal 3: To appoint Kesselman & Kesselman, a member of PricewaterhouseCoopers International Ltd., local time.as Teva’s independent registered public accounting firm until Teva’s 2022 annual meeting of shareholders.
Who may attend and
In addition, shareholders will consider Teva’s annual consolidated financial statements for the year ended December 31, 2020.
The Board of Directors recommends that you vote at the FOR all proposals.
Teva urges all of its shareholders to review its annual report (“Annual Meeting?
Attendance at the Annual Meeting will be limited to holders of record who hold ordinary shares or American Depositary Shares (“ADSsReport”), directly in their own name and beneficial owners who hold ordinary shares or ADSs through a broker, bank or other nominee rather than directly in their own name and each of their legal proxy holders or their authorized persons. To gain admission to on Form 10-K for the Annual Meeting, one must have a form of government-issued photograph identification and proof of share ownership as of the Record Date (as defined below). Legal proxy holders and authorized persons will also need to submit a document of appointment, in accordance with Teva’s Articles of Association.year ended December 31, 2020.
Holders of our mandatory convertible preferred shares do not have any voting rights or any other rights with respect to the Annual Meeting.
What is a quorum for the Annual Meeting?
A minimum of two Two holders of ordinary shares (or ADSs representing such ordinary shares) who are present at the Annual Meeting, in person or by proxy or represented by their authorized persons, and who hold in the aggregate twenty-five percent or more of such ordinary shares, (or ADSs representing such ordinary shares), willshall constitute a legal quorum. At the close of business on April 12, 2018, 1,018,220,001 ordinary shares were outstanding and entitled to vote. Ordinary shares held in treasury will not be included in the calculation to determine if a quorum is present. Abstentions and brokernon-votes will be considered present and entitled to vote for the purpose of determining the presence of a quorum. Should no legal quorum be present one halfone-half hour after the scheduled time, the Annual Meeting willshall be adjourned to one week from that day, at the same time and place. Should such legal quorum not be present one half hour after the time set for the Annual Meeting, as adjourned, any two holders of ordinary shares present, in person or by proxy, who jointly hold twenty percent or more of such ordinary shares (or ADSs representing ordinary shares) will then constitute a legal quorum.
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 1virtual format.
By Order of the Board of Directors,

Dov Bergwerk
Senior Vice President,
Company Secretary
April 21, 2021
Important Notice Regarding the Availability of Proxy Materials for the Annual Meeting to be Held on June 14, 2021
The accompanying Proxy Statement and our Annual Report are available at www.tevapharm.com/2021proxymaterials. We expect the proxy materials to be mailed and/or made available on or before April 30, 2021.
Teva Pharmaceutical Industries Ltd. | i | |
Questions and Answers About the Annual Meeting
Attendance at the Annual Meeting will not cause your previous vote to be revoked unless you specifically so request.
Proxy Materials
Why did I receive a “Notice of Internet Availability of Proxy Materials” but no proxy materials?
We distribute our Notice of Annual Meeting of Shareholders, Proxy Statement and Annual Report (collectively, the “proxy materials”) to certain shareholders via the Internet under the “Notice and Access” approach permitted by rules of the SEC. This approach conserves natural resources and reduces our distribution costs, while providing a timely and convenient method of accessing the materials and voting. On April 25, 2018, we mailed a “Notice of Internet Availability of Proxy Materials” to participating shareholders containing instructions on how to access the proxy materials on the Internet.
Can I access the proxy materials on the Internet?
The proxy materials are available on our website at www.tevapharm.com/2018proxymaterials. Information on our website is not part of the proxy materials and is not incorporated into the proxy statement by reference. Record owners of our ADSs may also access the proxy materials at www.proxydocs.com/teva by following the instructions provided by the Depositary. Beneficial owners of our ADSs may also access the proxy materials at www.proxyvote.com by following the instructions provided by your broker, bank or other nominee. Instead of receiving future proxy statements and accompanying materials by mail, most shareholders can elect to receive ane-mail that will provide electronic links to them. Opting to receive your proxy materials online will conserve natural resources and will save us the cost of producing documents and mailing them to you.The proxy materials are also available through Teva’s public filing on MAGNA (the Israeli Securities Authority’s electronic filing system) at www.magna.isa.gov.il, on the TASE’s website at www.maya.tase.co.il, or on the SEC’s website at www.sec.gov.
How do I request paper copies of the proxy materials at no charge?
You may contact Investor Relations in the United States at +1 (215)591-8912 or in Israel at +972 (3)926-7656, by sending an email to TevaIR@tevapharm.com, or by making a request on our website at www.tevapharm.com/InfoRequest, by May 22, 2018.
If you are a record owner of ADSs, you may request proxy materials at www.investorelections.com/teva, by calling (866)870-3684 or by sending an email to paper@investorelections.com, by May 22, 2018 and following the instructions provided by the Depositary.
If you are a beneficial owner of ADSs, you may request proxy materials by following the instructions at www.proxyvote.com, by calling (800)579-1639 or by sending an email to sendmaterial@proxyvote.com, by May 22, 2018 and following the instructions provided by your broker, bank or other nominee.
Other Questions
Could other matters be decided at the Annual Meeting?
The only items of business that our Board of Directors intends to present at the Meeting are set forth in this proxy statement. As of the date of this proxy statement, no shareholder has advised us of the intent to present any other matter, and we are not aware of any other matter to be presented at the Meeting. However, according and subject to the Israeli Companies Law and our Articles of Association, certain shareholders are entitled to propose items to the agenda. For more information, please see “Shareholder Proposals for the 2018 Annual Meeting and the 2019 Annual Meeting” below.
4 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Questions and Answers About the Annual Meeting
Who will pay for the cost of this proxy solicitation?
Teva will bear the entire cost of solicitation of proxies, including preparation, assembly, printing, and mailing of this proxy statement, the voting instruction card and any additional information furnished to shareholders. Teva may reimburse brokerage firms and other persons representing beneficial owners of ordinary shares or ADSs for reasonable expenses incurred by them in forwarding proxy soliciting materials to such beneficial owners. We retained MacKenzie Partners, Inc. to assist with the solicitation of proxies for a fee in the amount of $20,000, plus reimbursable expenses. In addition to solicitation by mail, certain of our directors, officers and regular employees, without additional remuneration, may solicit proxies by telephone, facsimile or personal contact.
Who can I contact if I require further assistance?
If you need assistance in submitting your proxy or have questions regarding the Annual Meeting, please contact our Investor Relations department by email at TevaIR@tevapharm.com or by mail at Teva Pharmaceutical Industries Ltd., 5 Basel Street, Petach Tikva, Israel, attention: Investor Relations or by telephone at +1(215) 591-8912. You may also contact our proxy solicitor, MacKenzie Partners, Inc., by email at proxy@mackenziepartners.com or by telephone (toll-free) at +1 (800)322-2885.
Teva is a leading global pharmaceutical company. In 2020, we continued helping patients around the world to access affordable medicines and benefit from innovations to improve their health. Despite the COVID-19 pandemic challenges, we saw minimal impact on our supply chain, R&D programs and product launches. Our key growth drivers delivered promising results and milestones, and we met all components of our 2020 financial guidance.
Looking ahead, we will continue to optimize our manufacturing network, portfolio and pipeline, improve our profitability and generate cash, as we remain on track to repay our debt and achieve our long-term financial targets.
2020 Financial Results
 |  |  | ||
| Revenue | EPS | Cash | ||
| $16.7 billion | $(3.64) (GAAP) | $1.2 billion (cash flow from operating activities) | ||
$2.57 (non-GAAP*) | $2.1 billion (free cash flow**) | |||
| * | For a reconciliation of non-GAAP EPS to GAAP EPS, see Teva’s press release filed on Form 8-K on February 10, 2021. |
| ** | Free cash flow includes cash flow generated from operating activities net of capital expenditures and deferred purchase price cash component collected for securitized trade receivables. For a reconciliation of free cash flow to cash flow from operating activities, see Teva’s press release filed on Form 8-K on February 10, 2021. |
Key Product Updates—Leveraging Our Growth Engines
AUSTEDO® Continued strong growth in the U.S. Launched in China in early 2021. Submissions continue in various other countries around the world. | AJOVY® Global sales bolstered by launch of auto-injector. Launched throughout the EU and in certain international markets. | Biosimilars Truxima® achieved ~24% of U.S. market share. We strengthened our pipeline with a new biosimilar commercialization agreement. | ||||||
Generics Launched first generic versions of HIV-1 treatments Truvada® and Atripla® in the U.S. Launched generic version of NuvaRing® in the U.S. in January 2021. | Digihaler® Launched three digital inhalers in the U.S.: ProAir®, ArmonAir® and AirDuo® with built-in sensors that track inhaler events and measure inspiratory flow. | Risperidone LAI Announced positive phase 3 results for risperidone LAI for patients with schizophrenia in January 2021. |
| Teva Pharmaceutical Industries Ltd. | 1 | |
2020 Overview
Teva’s Response to COVID-19
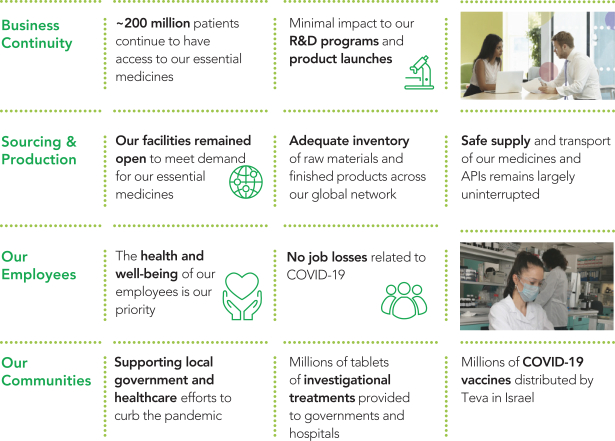
For further details, see “Item 7—Management’s Discussion and Analysis of Financial Condition and Results of Operations—The COVID-19 Pandemic” in our Annual Report on Form 10-K for the year ended December 31, 2020.
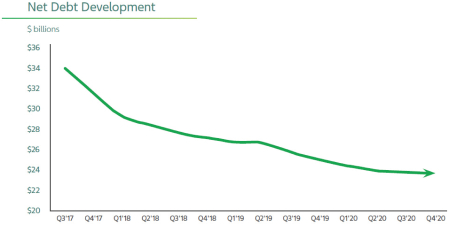
Continuing to Reduce our Debt
Proposal 1: Election of Directors
 | In |
| 2 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Board and Corporate Governance Highlights
Our Board of Directors (the “Board of Directors” or the “Board”) continually evaluates Teva’s corporate governance policies and practices, focusing on ensuring effective oversight of Teva’s business and management. We have established a strong and effective framework to monitor the risks of our business.
Board and Corporate Governance
| ∎ | Board refreshment and succession planning—seven new directors appointed over the last five years |
| ∎ | 11 out of |
| ∎ | All members of our committees are independent |
| ∎ | 25% female directors serving on the Board |
| ∎ | Annual Board and committees evaluation process |
Board Oversight of Risk
| ∎ | Full Board and individual Committees focus on understanding and assessing Company risks, including the oversight of risks related to the COVID-19 pandemic |
| ∎ | Board reviews risk management policies of our operations and business strategy and Board committees review risk in their areas of expertise |
| ∎ | The Audit Committee assists the Board with |
| ∎ | The Compliance Committee oversees our policies and practices for legal, regulatory and internal compliance (other than regarding financial reporting), our strategy and governance of |
| ∎ | The Finance and Investment Committee reviews our financial risk management policies, including |
| ∎ | The Human Resources and Compensation Committee (the “HR and Compensation Committee”) oversees compensation, retention, succession and other human resources-related issues and risks, as well as initiatives to promote inclusion and diversity |
| ∎ | The Science and Technology Committee oversees risks relating to our intellectual property and research and development activities |
| ∎ | The Corporate Governance and Nominating Committee oversees risks relating to governance policies and initiatives |
Director Alignment with Shareholder Interests
| ∎ | In 2020, directors had an outstanding meeting attendance rate of 100% |
| ∎ | We maintain director stock ownership guidelines requiring stock ownership of five times the annual cash fee (excluding committees fees) paid to directors, which must be achieved within a certain timeframe |
Shareholder Engagement
| ∎ | Active shareholder engagement efforts, led by our Chairman of the Board and other Board members. Discussions are focused on responding to feedback received from shareholders on corporate governance, executive compensation and ESG matters |
Our Environmental, Social and Governance Priorities and Accomplishments
| ∎ | In 2020, Teva renewed its ESG strategy, which is core to our business and reflects how we address environmental and social issues, while also bringing value to Teva. Our assessment identified topics that we believe matter most to our stakeholders and our business, including access to health and medicines, inclusion and diversity, ethics and climate action and resilience |
| ∎ | Teva set new long-term environmental targets to help advance climate action and resilience, responsible use of |
| ∎ | We continue to |
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 3 | |
Proposal 1: Election of Directors
We continue our efforts to attract the highest quality talent to our Board, by adding diverse and highly qualified directors with global pharmaceutical experience and other qualifications.
Dr. Barer, our Chairman of the Board, is an independent director under NYSE regulations. Kåre Schultz, our President and Chief Executive Officer (the “President and CEO”) serves on the Board, which facilitates collaboration between the Board of Directors and management. Corporate governance remains a high priority and we continue to evaluate the size and composition of the Board to ensure that it maintains dynamic, exceptionally qualified leadership.
Following the recommendation of our Corporate Governance and Nominating Committee, the Board of Directors recommends that shareholders approve the appointment of Ms.Rosemary A. Crane, Mr.Abbas Hussain, Gerald M. Lieberman and Prof. Ronit Satchi-Fainaro to serve as directors to serve until our 20212024 annual meeting of shareholders. Ms. Crane and Mr. LiebermanAll nominees are currently members of the Board of Directors and all nominees qualify as independent directors under NYSE regulations.
In accordance with the Israeli Companies law, 5759-1999 (as amended from time to time, the “Israeli Companies Law”), all nominees for election as directors at the Annual Meeting have declared in writing that they possess the requisite skills and expertise, as well as sufficient time, to perform their duties as directors.
|
The Board of Directors recommends that shareholders vote FOR the appointment of Rosemary A. Crane, Abbas Hussain, Gerald M. Lieberman and Prof. Ronit Satchi-Fainaro, |
The following table sets forth information regarding the directors and director nominees of Teva as of April 10, 2021:
Name | Age | Director Since | Term Ends | |||||||||
Dr. Sol J. Barer—Chairman | 73 | 2015 | 2023 | |||||||||
Kåre Schultz | 59 | 2017 | (1) | |||||||||
Rosemary A. Crane | 61 | 2015 | 2021 | |||||||||
Amir Elstein | 65 | 2009 | 2022 | |||||||||
Jean-Michel Halfon | 69 | 2014 | 2023 | |||||||||
Abbas Hussain (2) | 56 | 2020 | 2021 | |||||||||
Gerald M. Lieberman | 74 | 2015 | 2021 | |||||||||
Roberto A. Mignone | 49 | 2017 | 2022 | |||||||||
Dr. Perry D. Nisen | 65 | 2017 | 2022 | |||||||||
Nechemia (Chemi) J. Peres | 62 | 2017 | 2023 | |||||||||
Prof. Ronit Satchi-Fainaro | 49 | 2018 | 2021 | |||||||||
Janet S. Vergis | 56 | 2020 | 2023 | |||||||||
6 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
| (1) | Mr. Schultz’s term ends contemporaneously with his term as President and CEO. |
| (2) | Mr. Hussain was appointed in September 2020 by the Board to serve until the Annual Meeting, where his nomination will be presented to shareholders for approval. |
| 4 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Proposal 1: Election of Directors
Directors
The following table sets forth information regarding the directors and director nominee of Teva as of April 25, 2018:
Name | Age | Director Since | Term Ends | ||||||||||||
Dr. Sol J. Barer—Chairman
|
| 71
|
|
| 2015
|
|
| 2020
|
| ||||||
Kåre Schultz (1)
|
| 56
|
|
| 2017
|
|
| (2
| )
| ||||||
Rosemary A. Crane
|
| 58
|
|
| 2015
|
|
| 2018
|
| ||||||
Amir Elstein
|
| 62
|
|
| 2009
|
|
| 2019
|
| ||||||
Murray A. Goldberg
|
| 73
|
|
| 2017
|
|
| 2020
|
| ||||||
Jean-Michel Halfon
|
| 66
|
|
| 2014
|
|
| 2020
|
| ||||||
Gerald M. Lieberman
|
| 71
|
|
| 2015
|
|
| 2018
|
| ||||||
Galia Maor*
|
| 75
|
|
| 2012
|
|
| 2018
|
| ||||||
Roberto A. Mignone
|
| 46
|
|
| 2017
|
|
| 2019
|
| ||||||
Dr. Perry D. Nisen
|
| 62
|
|
| 2017
|
|
| 2019
|
| ||||||
Nechemia (Chemi) J. Peres
|
| 59
|
|
| 2017
|
|
| 2020
|
| ||||||
Prof. Ronit Satchi-Fainaro
|
| 46
|
|
| —
|
|
| —
|
| ||||||
Dan S. Suesskind*
|
| 74
|
|
| 2017
|
|
| (3
| )
| ||||||
Gabrielle Sulzberger*
|
| 57
|
|
| 2015
|
|
| 2018
|
| ||||||
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 7
Proposal 1: Election of Directors
Persons Being Considered for Election at thisthe Annual Meeting
Rosemary A. Crane Independent Director
Committees: –Human Resources andCompensation (Chair) –Science and Technology | Ms. Crane joined the Board of Directors in
| |
Qualifications:
With over 30 years of experience in commercialization and business operations, primarily in the pharmaceutical and
| ||
Abbas Hussain Independent Director Committees: – Audit – Finance and Investment – Science and Technology | Mr. Hussain joined the Board of Directors in September 2020. Mr. Hussain has served on the board of directors of Cochlear Limited and CSL Limited, both since 2018. From 2008 to 2017, Mr. Hussain held senior executive positions at GlaxoSmithKline plc (“GSK”), including Global President, Pharmaceuticals and Vaccines from 2013 to 2017, President, Europe and Emerging Markets, Pharmaceuticals from 2011 to 2013 and President, Emerging Markets, Pharmaceuticals from 2008 to 2011. Prior to joining GSK, he held senior roles with global responsibility at Eli Lilly and Company from 1998 to 2008, including President, European Operations from 2006 to 2008. Mr. Hussain has Joint Honors in medicinal chemistry and pharmacology from Loughborough Institute of Technology. | |
Qualifications: With his executive experience in the biopharmaceutical industry and deep biotechnology insight, and through his executive and non-executive roles, Mr. Hussain will provide the Board with a broad global perspective and understanding of pharmaceutical manufacturing, product development, risk, health, safety, environment and corporate responsibility. |
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 5 | |
Proposal 1: Election of Directors
Gerald M. Lieberman Independent Director
Committees: –Audit (Chair) –Human Resources and Compensation – Finance and Investment | Mr. Lieberman joined the Board of Directors in
| |
Qualifications:
With his many years of experience as an executive in leading financial services companies, including his knowledge and experience in human capital development, succession planning and compensation, Mr. Lieberman provides finance, risk management, operating and
|
8 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Proposal 1: Election of Directors
Prof. Ronit Satchi-Fainaro Independent Director Committees: – Science and Technology – Compliance | Prof. Satchi-Fainaro
| |
Qualifications:
With extensive experience in clinical medicine and research, Prof. Satchi-Fainaro providesin-depth knowledge of medicine and
|
As required by Israeli law, all
| 6 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Proposal 1: Election of the foregoing director candidates have declared in writing that they possess the requisite skills and expertise, as well as sufficient time, to perform their duties as a director.Directors
Dr. Sol J. Barer Chairman of the Board Independent Director | Dr. Barer became Chairman of the Board of Directors
| |
Qualifications:
With his long career as a senior pharmaceutical executive and leadership roles in various biopharmaceutical companies, Dr. Barer provides broad and experienced knowledge of the global pharmaceutical business and industry as well as extensive scientific expertise.
| ||
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 9
Proposal 1: Election of Directors
Kåre Schultz Director and President and Chief Executive Officer | Mr. Schultz became Teva’s President and CEO and a member of the Board of Directors on November 1, 2017. From May 2015 to October 2017, Mr. Schultz served as President and Chief Executive Officer of H. Lundbeck A/S. Prior to that, Mr. Schultz worked for nearly three decades at Novo Nordisk, where he served in a number of leadership roles, including Chief Operating Officer, Vice President
| |
Qualifications:
|
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 7 | |
Proposal 1: Election of Directors
Amir Elstein Independent Director
Committees: – Corporate Governance and Nominating (Chair) –Audit –Finance and Investment | Mr. Elstein rejoined the Board of Directors in 2009. From January 2014 to July 2014, he served as Vice Chairman of the Board of Directors of Teva. Mr. Elstein
| |
Qualifications:
|
10 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Proposal 1: Election of Directors
|
| |
|
Jean-Michel Halfon Independent Director
Committees: –Compliance (Chair) –
| Mr. Halfon joined the Board of Directors in 2014. He currently serves as an independent consultant, providing consulting services to pharmaceutical, distribution, healthcare IT and R&D companies. From 2008 to 2010, Mr. Halfon served as President and General Manager of Emerging Markets at Pfizer Inc., after serving in various senior management positions since 1989. From 1987 to 1989, Mr. Halfon served as Director of Marketing in France for Merck & Co., Inc. Mr. Halfon received a B.S. from Ecole Centrale des Arts et Manufactures and an M.B.A. from Institut Supérieur des Affaires. | |
Qualifications:
|
| 8 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 11
Proposal 1: Election of Directors
Roberto A. Mignone Independent Director
Committees: – Finance andInvestment (Chair) – –Corporate Governanceand | Mr. Mignone joined the Board of Directors in
| |
Qualifications:
With his long career as a global investment professional
|
Dr. Perry D. Nisen Independent Director
Committees: –Science – Compliance | Dr. Nisen joined the Board of Directors in
| |
Qualifications:
|
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 9 | |
12 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Proposal 1: Election of Directors
Nechemia (Chemi) J. Peres Independent Director
Committees: –Corporate Governanceand Nominating –Human Resources and Compensation | Mr. Peres joined the Board of Directors in
| |
Qualifications:
With his pioneering financial and entrepreneurial background, Mr. Peres provides the Board with a forward-thinking view on financial and strategic matters.
|
Janet S. Vergis Independent Director Committees: – Human Resources and Compensation – Compliance | Ms. Vergis joined the Board of Directors in June 2020. She served as a retained executive advisor to various private equity firms from 2013 to 2019. From 2011 to 2012, she served as the Chief Executive Officer of OraPharma, Inc., a specialty pharmaceutical company. From 2004 to 2009, she served as President of Janssen Pharmaceuticals LP, McNeil Pediatrics, Inc. and Ortho-McNeil Neurologics, Inc., subsidiaries of Johnson & Johnson. Ms. Vergis contributed to a number of Johnson & Johnson companies during her career, holding positions of increasing responsibility in research and development, new product development, sales and marketing. Ms. Vergis has served on the board of directors of Church and Dwight Co., Inc. since 2014, Dentsply-Sirona, Inc. since 2019 and SGS SA since March 2021. She previously served on the board of directors of MedDay Pharmaceuticals from 2016 to 2021, Amneal Pharmaceutical from 2015 to 2019, Lumara Health from 2013 to 2014 and OraPharma, Inc. from 2011 to 2012. Ms. Vergis received a Bachelor of Science in Biology and a Master’s of Science in Physiology from The Pennsylvania State University. | |
Qualifications: With over 30 years of experience in many aspects of the healthcare industry, including research and development, new product development, sales, and various executive roles, as well as her experience as a board member of public pharmaceutical companies, Ms. Vergis provides the Board with broad global business experience in the pharmaceutical industry. |
Directors whose Service is Concluding at the Meeting
After two terms of service, Galia Maor has decided not to submit her candidacy for reelection at the Annual Meeting. Gabrielle Sulzberger and Dan S. Suesskind have decided not to seek reelection after one term and approximately one year of service, respectively. We sincerely thank them for their contribution, leadership and critical efforts on behalf of Teva throughout their respective terms of service. The Board will miss their insight and perspective in its deliberations and hope they will remain close to Teva for years to come.
Family Relationships
There are no family relationships among any of our executive officers or directors.
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 13
| 10 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Corporate Governance and Director Compensation
Board Practices
Under our Articles of Association, the Board of Directors must consist of between three to 18 directors (including our President and CEO and two statutory independent directors, if required). Our Board of Directors currently consists of 13 persons, including our President and CEO. Subject to election of all of the directors included in Proposal 1, and following the departure of the above-mentioned directors, our Board of Directors will consist of 1112 persons, including our President and CEO. The Board of Directors has determined that all of the directors that currently serve on the Board of Directors, all of the directors that served on the Board during 2017 and all of the directors that will serve on the Board subject to their election atof Directors following the Annual Meeting were and are as applicable, independent, except for Kåre Schultz, Erez Vigodman and Dr. Yitzhak Peterburg, each of whom served, or in the case of Mr. Schultz serves, on the Board of Directors while serving as our President and CEO (or as interim President and Chief Executive Officer, in the case of Dr. Peterburg).CEO.
We currently maintain a policy to have at least threetwo directors qualify as financial and accounting experts under Israeli law. Accordingly, the Board of Directors has determined that of the continuing directors, Murray A. Goldberg, Gerald M. Lieberman and Roberto A. Mignone are financial and accounting experts under such criteria.
Our directors are generally entitled to review and retain copies of our documentation and examine our assets, as required to perform their duties as directors and to receive assistance, in special cases, from outside experts at our expense.
Board Diversity*Diversity and Skills
Over the course of 2020, inclusion and diversity was a point of emphasis for our Board and our management team. Teva believes that inclusion and diversity are essential to its ability to innovate and grow its business. It is our desire to create and sustain an inclusive and diverse work environment.
  |   |   |
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 11 | |
Corporate Governance and Director Compensation
The chart below summarizes the notable skills, qualifications and experience of each of our directors and director nominees (in addition to requisite skills and expertise to perform their duties as directors) and highlights the balanced mix of skills, qualifications and experience of the Board as a whole. These are the same attributes that the Board considers as part of its ongoing director succession planning process. This high-level summary is not intended to be an exhaustive list of each director’s and director nominee’s skills or contributions to the Board.
SKILLS/QUALIFICATIONS/ | S. | K. | R. | A. | J. M. | A. | G. | R. | P. | N. | R. | J. | ||||||||||||
Accounting and financial reporting experience | ✓ | ✓ | ✓ | |||||||||||||||||||||
CEO / executive management leadership skills | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
Human resource management and executive comp. knowledge and experience | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||
Pharmaceutical industry | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
Commercial and operations management | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||||
Risk oversight and risk management | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||
Science / medical | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||
Finance and investment markets | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||||
ESG | ✓ | ✓ | ✓ | |||||||||||||||||||||
Academia/Education | ✓ | ✓ | ||||||||||||||||||||||
Global perspective, international | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
ADDITIONAL QUALIFICATIONS AND INFORMATION | ||||||||||||||||||||||||
Audit committee financial expert / financial expert under Israeli law | ✓ | ✓ | ||||||||||||||||||||||
Other public boards | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||||
Director Terms and Education. Our directors are generally elected in three classes for terms of approximately three years. Due to the complexity of our businesses and our extensive global activities, we value the insight and familiarity with our operations that a director is able to develop over his or her service on the Board of Directors. Because we believe that extended service on our Board enhances a director’s ability to make significant contributions to Teva, we do not believe that arbitrary term limits on directors’ service are appropriate. At the same time, it is the policy of the Board that directors should not expect to be renominated automatically.
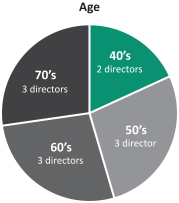
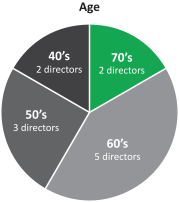
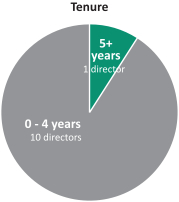
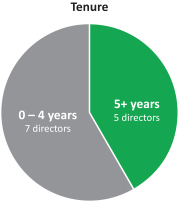
In recent years, we strengthened our Board of Directors with the addition of new highly qualified and talented directors, adding expertise as well as diversity to our Board of Directors. Through these efforts, we have reduced theThe average tenure of our directors from 5.1 years of service prior to the 2017 annual meeting of shareholders to 2.5 years after giving effect to all nominations and departures contemplated
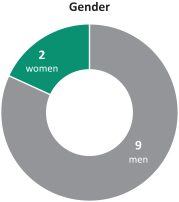
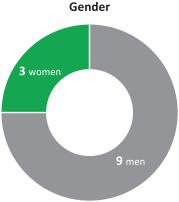
Age Tenure Gender 5+ 40’s years 2 1 directors 2 directors women 70’s 3 directors 0 - 4 years 50’s 10 directors 60’s 3 director 9 3 directors men
14 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
| 12 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Corporate Governance and Director Compensation
herein. We also reducedcurrent directors is 4.8 years of service and the average age is 61.5 years. We currently have three female directors out of 12 members serving on our directors from 67 prior to the 2017 annual meetingBoard of shareholders to 61 after giving effect to all nominations and departures contemplated herein.Directors. Our Chairman of the Board is independent under NYSE regulations, and 1211 out of 13of12of our current directors are and following the election of all of the directors included in Proposal 1 and the departure of the above-mentioned directors 10 out of 11 of the directors will be, independent under NYSE regulations. Our onlynon-independent director is our President and CEO, which facilitates collaboration between the Board of Directors and management. We continue to evaluate the size and composition of our Board of Directors to ensure it maintains dynamic, exceptionally qualified members.
We provide an orientation program and a continuing education process for our directors, which include business and industry briefings, provision of materials, sessions from leading experts and professionals, meetings with key management and visits to Teva facilities. We evaluate and improve our education and orientation programs on an ongoing basis to ensure that our directors have the knowledge and background needed for them to best perform their duties.
Board Meetings. The Board of Directors holds at least six meetings each year to review significant developments affecting Teva and to consider matters requiring approval of the Board, with additional meetings scheduled when important matters require Board of Directors action between scheduled meetings. A majorityIn consideration of the health and safety of our directors, executive officers and other employees, our Board and Committees meetings convened, but not fewer than four, must bewere conducted virtually in Israel.2020, due to the COVID-19 pandemic. Members of senior management regularly attend Board meetings to report on and discuss their areas of responsibility. Information regarding the number of Board committee meetings and attendance rates for 20172020 is presented in the table on page 20.below under “—Committee Composition and Board and Committee Attendance in 2020.”
Executive Sessions of the Board. Our directors meet in executive session (i.e., without the presence of management, including our President and CEO) generally in connection with each regularly scheduled Board meeting and additionally as needed. Executive sessions are chaired by Dr. Barer, the Chairman of the Board.
Annual Meetings. We do not have a formal policy requiring members of the Board to attend our annual meetings, although all directors are strongly encouraged to attend. NineAll of our directors attended the 20172020 annual meeting of shareholders.shareholders, which was held virtually.
Board Leadership.The Board of Directors recognizes that one of its key responsibilities is to establish and evaluate an appropriate leadership structure for the Board of Directors so as to provide effective oversight of management. The Board of Directors has separate roles for the Chief Executive Officer and Chairman of the Board of Directors, with Dr. Sol Barer serving as independent Chairman and Mr. Kåre Schultz as President and CEO. Dr. Barer’s long career as a senior pharmaceutical executive and leadership roles in various biopharmaceutical companies, as well as his extensive scientific expertise and knowledge of the global pharmaceutical business, have made him an invaluable resource to both the Board of Directors and the Chief Executive Officer. The Board of Directors has determined that this leadership structure is appropriate for Teva at this time.time because it ensures that the appropriate level of oversight, independence and responsibility is applied to all Board decisions.
Board of Directors Role in Risk Oversight. Management is responsible for assessing and managing risk, subject to oversight by the Board of Directors. Our annual risk assessment process includes both atop-down review of strategic risks and abottom-up review of operational risks, which are presented to the Board of Directors. The Board of Directors fulfills its oversight responsibility for risk assessment and management by reviewing risk management policies and the risk appetite of our operations and business strategy and by instructing its committees to assist and advise in their areas of expertise, as described below. Each committee provides regular updates to the full Board regarding its activities.
| ∎ | The Board oversees our risk management policies and risk appetite, including operational risks and risks relating to our business strategy and transactions. Various committees of the Board assist the Board in this oversight responsibility in their respective areas of expertise. |
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 15
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 13 | |
Corporate Governance and Director Compensation
| ∎ | The Audit Committee assists the Board with the oversight of our financial reporting, independent auditors, internal controls, |
| ∎ | The Compliance Committee oversees our policies and practices for legal, regulatory and internal compliance (other than regarding financial reporting) and reviews policies and practices that may seriously impact our reputation. |
| ∎ | The Finance and Investment Committee reviews our financial risk management policies, including our investment guidelines, financings and foreign exchange and currency hedging, as well as financial risk of certain transactions. |
| ∎ | The |
| ∎ | The Science and Technology Committee oversees risks relating to our intellectual property and research and development activities. |
| ∎ | The Corporate Governance and Nominating Committee oversees risks relating to our governance policies and initiatives. |
During 2020, the Board and the HR and Compensation Committee closely monitored our performance in light of the COVID-19 pandemic. This included review of the effects the COVID-19 pandemic had on our business performance and operations, as well as on the safety, morale and engagement of our employees.
Cybersecurity Risk Management.The Audit Committee assists the Board with the oversight of cybersecurity risks. As part of its risk oversight function, the Audit Committee reviews our cyber risk assessment and management policies and receives briefings concerning Teva’s information security and technology risks, including cybersecurity. During 2020, the Audit Committee received four periodic briefings on Teva’s information security and risk management programs, including with respect to cyber security, global cyber threat trends, SAP implementation, threats arising from the COVID-19 pandemic and privacy controls. Teva’s information security office leads our cybersecurity risk management program. We also maintain cyber risk insurance coverage.
Director Service Contracts. Except for equity awards that accelerate upon termination, we do not have any contracts with any of ournon-employee directors that provide for benefits upon termination of services. Information regarding director compensation can be found under“Non-Employee Director Compensation” below.
Communications with the Board. Shareholders, employees and other interested parties can contact any director or committee of the Board of Directors by writing to them care of Teva Pharmaceutical Industries Ltd., 5 Basel124 Dvora HaNevi’a Street, Petach Tikva, 4951033,Tel Aviv, 6944020, Israel, Attn: Company Secretary or Internal Auditor.Auditor or by email to TevaAGM2021@tevapharm.com. Comments or complaints relating to our accounting, internal controls or auditing matters may also be referred to members of the Audit Committee, as well as other appropriate Teva bodies.departments. The Board of Directors has adopted a global “whistleblower” policy, which provides employees and others with an anonymous means of communicating with the Audit Committee.
Nominees for Directors. In accordance with the Israeli Companies Law, a nominee for service as a director must submit a declaration to us, prior to his or her election, specifying that he or she has the requisite qualifications to serve as a director and the ability to devote the appropriate time to performing his or her duties as such and that he or she is not restricted from serving as director under the Israeli Companies Law. All of our directors, including those nominated for appointment as directors at the Annual Meeting, have provided such declaration. A director who ceases to meet the statutory requirements to serve as a director must notify us to that effect immediately and his or her service as a director will terminate upon submission of such notice.
| 14 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Corporate Governance and Director Compensation
Our Board of Directors believes that it should be composed of directors with diverse, complementary backgrounds and that directors should, at a minimum, exhibit proven leadership capabilities and possess experience at a high level of responsibility within their chosen fields. When considering a candidate for director, our Corporate Governance and Nominating Committee considers whether the directors, both individually and collectively, can and do provide the experience, judgment, commitment, skills and expertise appropriate to lead Teva in the context of its industry. In addition, our Corporate Governance and Nominating Committee considers a nominee’s expected contribution to the diversity of skills, background, experiences and perspectives, as well as whether such nominee could provide added value to any of the committees of the Board of Directors, given the then existing composition of the Board of Directors as a whole. When seeking new candidates, the Corporate Governance and Nominating Committee also considers candidates representing a diversity of backgrounds, perspectives, ethnicities, races and genders. Our Corporate Governance and Nominating Committee also provides input and guidance regarding the independence of directors, for formal review and approval by our Board of Directors.
16 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Corporate Governance and Director Compensation
When seeking candidates for director,directorships, our Corporate Governance and Nominating Committee may solicit suggestions from incumbent directors, management, shareholders and others. Additionally, the Board of Directors has in the past used and may continue to use the services of third party search firms to assist in the identification and analysis of appropriate candidates. After conducting an initial evaluation of a prospective candidate, members of the Board of Directors will interview that candidate if they believe the candidate may be suitable. The Chairman of the Board of Directors may also ask the candidate to meet with certain members of executive management.
If our Corporate Governance and Nominating Committee believes a director should bere-approved or a candidate would be a valuable addition to the Board of Directors, it may recommend to the Board of Directors that candidate’s appointment or election, who, in turn, can submit the candidate for consideration by the shareholders.
The Israeli lawCompanies Law provides a process by which one or more shareholders holding 1% or more of the voting rights of Teva may propose the nomination of a candidate to the Board of Directors for consideration by Teva’s Corporate Governance and Nominating Committee.Directors. See “Shareholder Proposals for the 20182021 Annual Meeting and the 20192022 Annual Meeting” below.
Non-Employee Director Compensation
As required by the Israeli Companies Law, we have adopted a Compensation Policy for Executive Officers and Directors (the “Compensation Policy”), which is presented for shareholder approval at least once every three years.Pursuantyears.Pursuant to the Israeli Companies Law and regulations promulgated thereunder, any arrangement between Teva and a director relating to his or her compensation as a director or other position with Teva must generally be consistent with Teva’s Compensation Policy and approved by the HR and Compensation Committee, the Board and by a simple majority of Teva’s shareholders. Shareholder approval is not required in certain instances, for example, for the compensation granted to a director for the period following his or her appointment until the next general meeting of shareholders, provided such compensation is approved by the Compensation Committee and the Board, is consistent with the Compensation Policy and is on similar or less favorable terms than those of such person’s predecessor.
As approved at our 20152019 annual general meeting of shareholders, each of ournon-employee director annual compensation program (applicable to all non-employee directors from time to time (other than ourexcept for the Chairman of the Board) is entitled to annual compensation comprised of:
| (i) | an annual Board membership fee of |
| (ii) | additional annual cash fees for service on Board |
$20,000 per annum to serve as a member of the |
as approved at our 2017 annual general meeting of shareholders,
$15,000 per annum to serve as a member of the HR and Compensation Committee; and $30,000 per annum to serve as chairperson of the HR and Compensation Committee; |
| c. | $20,000 per annum to serve as a member on |
Our 2017 annual general meeting of shareholders approved an annual fee of $567,000 for our Chairman of the Board. This fee is in addition to the annual equity-based award in the form of RSUs our Chairman is entitled to with an approximate fair market value of $378,000 on the date of grant, as approved at our 2015 annual general meeting of shareholders. Our Chairman is also entitled to certain secretarial and other services and benefits.
All of our current directors waived 50% of the cash component of his or her annual Board membership fee, effective as of January 1, 2018 and until December 31, 2018. Giving effect to such waiver, the annual cash fee for their Board membership in 2018 will be $80,000.
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 17
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 15 | |
Corporate Governance and Director Compensation
| d. | $10,000 per annum to serve as a member of any other standing Board committee that is not listed in sub-sections (a)-(b); and $20,000 per annum to serve as chairperson on such committee; and |
| (iii) | an annual equity-based award in the form of restricted share units (“RSUs”) with an approximate aggregate grant date fair value of $160,000 and a one year cliff vesting. |
As approved at our 2019 annual general meeting of shareholders, the annual compensation for the Chairman of the Board is comprised of:
| (i) | an annual Board membership fee of $255,000 paid in cash; |
| (ii) | an annual equity-based award in the form of RSUs with an approximate aggregate grant date fair value of $285,000 and a one year cliff vesting; and |
| (iii) | office and secretarial services at Teva’s offices. |
The Chairman of the Board is not entitled to additional annual cash fees for service on Board Committees.
Fees for Board and committee service are payable over the period of time during which the individual serves as anon-employee director. In the event that anon-employee director serves as a member of the Board during only a portionpart of the period from one annual meeting to the next,year, apro-rated amount of the annual board membership fee and standing committee fees and equity award will be paid. In the event of an appointment to the Board between annual meetings of shareholders, the annual equity-based award shall be pro-rated.Upon completion of anon-employee director’s service as a director, other than removal pursuant to a shareholder resolution due to a breach of fiduciary duties, any unvested awards granted to such director inby virtue of such position and held by such director will immediately become vested.
In addition, Teva reimburses or covers its directors for expenses (including travel expenses) incurred in connection with meetings of the Board and its committees or performing other services for Teva in their capacity as directors, in accordance with Israeli law and the Compensation Policy. Directors, including the Chairman of the Board, are also entitled to certain perquisites having an aggregate monetary value of no more than $10,000 per year per director.
VAT, if applicable, is added to the above director compensation, in accordance with applicable law.
No additional compensation is received for attendance at a Board or Committee meeting.
2017 Director Compensation
Name | Fees Earned or Paid in Cash ($) (1) | Stock Awards ($) (2) | Total ($) | ||||||||||||
Dr. Sol J. Barer (3) | 243,389 | 454,884 | 698,273 | ||||||||||||
Roger Abravanel (4) | 104,445 | 0 | 104,445 | ||||||||||||
Dr. Arie Belldegrun (5) | 12,110 | 0 | 12,110 | ||||||||||||
Rosemary A. Crane | 201,681 | 130,001 | 331,682 | ||||||||||||
Amir Elstein | 190,592 | 130,001 | 320,593 | ||||||||||||
Murray A. Goldberg (6) | 85,957 | 130,001 | 215,958 | ||||||||||||
Jean-Michel Halfon | 198,116 | 130,001 | 328,117 | ||||||||||||
Gerald M. Lieberman | 208,343 | 130,001 | 338,344 | ||||||||||||
Galia Maor | 196,417 | 130,001 | 326,418 | ||||||||||||
Roberto A. Mignone (6) | 81,712 | 130,001 | 211,713 | ||||||||||||
Dr. Perry D. Nisen (6) | 81,712 | 130,001 | 211,713 | ||||||||||||
Joseph Nitzani (7) | 163,397 | 32,500 | 195,897 | ||||||||||||
Nechemia J. Peres (6) | 83,753 | 130,001 | 213,754 | ||||||||||||
Ory Slonim (8) | 106,103 | 0 | 106,103 | ||||||||||||
Dan S. Suesskind (9) | 50,357 | 103,889 | 154,246 | ||||||||||||
Gabrielle Sulzberger | 196,788 | 130,001 | 326,789 | ||||||||||||
|
18 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Corporate Governance and Director Compensation
Mr. Schultz was not and will not be entitled to any compensation in his capacity as a member of the Board or any committee thereof.
We purchase directors’ and officers’ liability insurance for our directors and executive officers, as approved by our shareholders and consistent with the Compensation Policy. In addition, we release our directors from liability and indemnify them to the fullest extent permitted by law and our Articles of Association, and provide them with indemnification and release agreements for this purpose, substantially in the form approved by our shareholders at our 2012 annual meeting.
In addition, Teva reimburses or covers its non-employee directors’ expenses (including travel expenses) incurred in connection with attending meetings of the Board and its committees or in performing other services for Teva in their capacity as non-employee directors, in accordance with Israeli law and the Compensation Policy.
Any director elected at the Meeting wouldto serve as a member of our Board and all directors currently serving on our Board will be remuneratedcompensated in the manner described above and wouldwill benefit from the insurance, indemnification and release discussed above.
No additional compensation is received for attendance at a Board or committee meeting.
Director Stock Ownership Guidelines
In 2019, we established director stock ownership guidelines requiring ownership of five times the annual cash fee paid to directors for board membership (excluding committees fees), which must be achieved within the later of six years of first becoming subject to these guidelines and January 1, 2025.
| 16 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Corporate Governance and Director Compensation
Name | Fees Earned or Paid in Cash ($) (1) | Stock Awards ($) (2) | Total ($) | ||||||||||||
Dr. Sol J. Barer (3) | 255,000 | 284,996 | 539,996 | ||||||||||||
Rosemary A. Crane | 170,000 | 159,997 | 329,997 | ||||||||||||
Amir Elstein | 180,000 | 159,997 | 339,997 | ||||||||||||
Murray A. Goldberg (4) | 72,608 | 0 | 72,608 | ||||||||||||
Jean-Michel Halfon | 160,000 | 159,997 | 319,997 | ||||||||||||
Abbas Hussain (5) | 50,000 | 122,670 | 172,670 | ||||||||||||
Gerald M. Lieberman | 195,000 | 159,997 | 354,997 | ||||||||||||
Roberto A. Mignone | 180,000 | 159,997 | 339,997 | ||||||||||||
Dr. Perry D. Nisen | 160,000 | 159,997 | 319,997 | ||||||||||||
Nechemia (Chemi) J. Peres | 155,000 | 159,997 | 314,997 | ||||||||||||
Prof. Ronit Satchi-Fainaro | 150,000 | 159,997 | 309,997 | ||||||||||||
Janet S. Vergis (6) | 86,007 | 159,997 | 246,004 | ||||||||||||
| (1) | The amounts shown include the paid cash portion of the annual fee for the Chairman of the Board and Board membership fees and committee service fees for other non-employee directors. |
| (2) | In June 2020, each non-employee director serving at that time was granted 12,668 RSUs, and the Chairman of the Board was granted 22,565 RSUs, based on the grant date fair value of a share of $12.63. Non-employee directors that join between annual general meetings are eligible for an equity grant value that is pro-rated in an amount equal to the difference between (i) an annual grant of $160,000 (for non-employee directors other than the chairman) and (ii) the product of (x) an annual grant ($160,000) divided by 12 and (y) the number of months (including partial months) in the period between the last annual meeting of shareholders and the date of such appointment. Accordingly, in September 2020, Abbas Hussain was granted 13,050 RSUs based on the grant date fair value of a share of $9.40. The amounts shown in the Stock Awards column represent the aggregate grant date fair values of RSUs computed in accordance with FASB Accounting Standards Codification Topic 718 (“Topic 718”). Valuations of RSUs were determined based on the fair market value of a Teva share on the grant date, less the net present value of dividends, as relevant. For information regarding assumptions, factors and methodologies used in our computations pursuant to Topic 718, see note 14c. to our consolidated financial statements set forth in our Annual Report on Form 10-K for the year ended December 31, 2020. These RSUs vest one year from the grant date. As of December 31, 2020, the aggregate number of unvested RSUs held by each current non-employee director was as follows: Dr. Sol J. Barer: 40,130; Rosemary A. Crane: 18,709; Amir Elstein: 18,709; Jean-Michel Halfon: 18,709; Abbas Hussain: 13,050; Gerald M. Lieberman: 18,709; Roberto A. Mignone: 18,709; Dr. Perry D. Nisen: 18,709; Nechemia J. Peres: 18,709; Prof. Ronit Satchi-Fainaro: 18,709; and Janet S. Vergis: 12,668. Upon completion or termination of a non-employee director’s service as a director, other than removal pursuant to a shareholder resolution due to a breach of fiduciary duties, any unvested awards granted to such director in virtue of such position and held by such director will immediately become vested. In 2020, Murray A. Goldberg received accelerated vesting of equity in connection with his completion of Board service. |
| (3) | During his service as Chairman of the Board, Dr. Barer is entitled to an annual fee of $255,000 and an annual equity-based award with an approximate grant date fair value of $285,000. |
| (4) | Mr. Goldberg’s term expired in June 2020. |
| (5) | Mr. Hussain was appointed to the Board on September 1, 2020. |
| (6) | Ms. Vergis was elected to the Board at the 2020 annual meeting on June 9, 2020. |
Mr. Schultz was not and will not be entitled to any compensation in his capacity as a member of the Board or any committee thereof.
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 17 | |
Corporate Governance and Director Compensation
Our Articles of Association provide that the Board of Directors may delegate its powers to one or more committees as it deems appropriate to the extent such delegation is permitted under the Israeli Companies Law. The Board of Directors has appointed the standing committees listed below, as well asad-hoc committees appointed from time to time for specific purposes determined by the Board.
We have adopted charters for all of our standing committees, formalizing the committees’ procedures and duties. These committee charters are available on our website at www.tevapharm.com.
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 19
Corporate Governance and Director Compensation
Current Committee Composition and Board and Committee Attendance in 2020
Name
| Audit
| Human and
| Corporate
| Finance and
| Compliance
| Science and
| ||||||
Rosemary A. Crane
| Chair
| ∎
| ||||||||||
Amir Elstein
| ∎
| Chair
| ∎
| |||||||||
Murray A. Goldberg
| ∎
| ∎
| ||||||||||
Jean-Michel Halfon
| ∎
| ∎
| Chair
| |||||||||
Roberto A. Mignone
| ∎
| ∎
| ||||||||||
Dr. Perry D. Nisen
| ∎
| Chair
| ||||||||||
Nechemia (Chemi) J. Peres
| ∎
| ∎
| ||||||||||
Gerald M. Lieberman
| Chair
| ∎
| ||||||||||
Galia Maor*
| ∎
| Chair
| ||||||||||
Gabrielle Sulzberger*
| ∎
| ∎
| ||||||||||
Dan S. Suesskind*
| ∎
| ∎
| ||||||||||
No. of meetings in 2017
| 10
| 11
| 6
| 6
| 2
| 4
| ||||||
Average attendance rate
| 96%
| 92%
| 92%
| 97%
| 100%
| 95%
| ||||||
* Ms. Maor, Mr. Suesskind and Ms. Sulzberger have each decided not to submit their candidacy for reelection at the Annual Meeting.
Name | Audit | Human and | Corporate Governance and Nominating | Finance and | Compliance | Science and | ||||||
Rosemary A. Crane |
| Chair |
|
|
| ∎ | ||||||
Amir Elstein | ∎ |
| Chair | ∎ |
|
| ||||||
Jean-Michel Halfon |
|
| ∎ |
| Chair |
| ||||||
Abbas Hussain | ∎ |
|
| ∎ |
| ∎ | ||||||
Roberto A. Mignone | ∎ |
| ∎ | Chair |
|
| ||||||
Dr. Perry D. Nisen |
|
|
|
| ∎ | Chair | ||||||
Nechemia (Chemi) J. Peres |
| ∎ | ∎ |
|
|
| ||||||
Gerald M. Lieberman | Chair | ∎ |
| ∎ |
|
| ||||||
Prof. Ronit Satchi-Fainaro |
|
|
|
| ∎ | ∎ | ||||||
Janet S. Vergis |
| ∎ |
|
| ∎ |
| ||||||
No. of meetings in 2020 | 7 | 6 | 5 | 4 | 4 | 4 | ||||||
Average attendance rate | 100% | 100% | 100% | 100% | 100% | 100% | ||||||
In 2017,2020, our Board of Directors met 16 times, with an average attendance rate of 94%.10 times. In 2017,2020, each of our current directors attended at least 80%100% of the meetings of the Board and Board committees on which he or she served. In 2017,consideration of the health and safety of our directors, executive officers and other employees, our Board and Committees meetings were conducted virtually in 2020, due to the COVID-19 pandemic.
In addition to the above, during 2020, the Chairman of the Board of Directors and various Board committees met frequently to review and approve the important strategic activities throughout the year.
We had a Corporate Responsibility Committee which was dissolved effective as of September 1, 2017, when we established our Compliance Committee. The Corporate Responsibility Committee met twice during 2017 until dissolved, with an attendance rate of 90%.chairpersons held discussions focusing on pending litigation matters.
Audit Committee
The Israeli Companies Law mandatesrequires publicly held Israeli companies to appoint an audit committee. As a NYSE-listed company, Teva’s Audit Committee must be comprised solely of independent directors, as defined by the Securities and Exchange Commission (the “SEC”) and NYSE regulations.
The responsibilities of our Audit Committee include, among others: (a) identifying flaws in the management of our business and making recommendations to the Board of Directors as to how to correct them and providing for arrangements regarding employee complaints with respect thereto; (b) making determinations and considering providing approvals concerning certain related party transactions and certain actions involving conflicts of interest; (c) reviewing the internal auditor’s performance and approving the internal audit work program and examining our internal control structure and processes; (d) examining the independent auditor’s scope of work and fees and providing the corporate body responsible for determining the independent auditor’s fees with its recommendations;fees; and (e) providing for arrangements regarding employee complaints regarding questionable accounting or auditing matters and monitormonitoring compliance with and investigateinvestigating alleged violations and enforceenforcing provisions of the Company’sTeva’s Code of Conduct. Furthermore, the Audit Committee discusses the financial statements and the disclosure under “Management’s Discussion and Analysis of Financial Condition and Results of Operations” (the “MD&A”) and presents to the Board of Directors its recommendations with respect to the proposed financial statements and MD&A.
20 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
| 18 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Corporate Governance and Director Compensation
In accordance with the Sarbanes-Oxley Act and NYSE requirements, the Audit Committee is directly responsible for the appointment, compensation and oversight of the work of our independent auditors. In addition, the Audit Committee is responsible for assisting the Board of Directors in monitoring our financial statements, the effectiveness of our internal controls and our compliance with legal and regulatory requirements. The Audit Committee also discusses our policies with respect to risk assessment and risk management with respect toregarding financial reporting and risks that may be material to us and major legislative and regulatory developments that could materially impact Teva’s contingent liabilities and risks.
The Audit Committee charter sets forth the scope of the committee’s responsibilities, including its structure, processes and membership requirements; the committee’s purpose; its specific responsibilities and authority with respect to, among others, registered public accounting firms; complaints relating to accounting, internal accounting controls or auditing matters; and its authority to engage advisors as determined by the Audit Committee.
The Audit Committee also reviews and receives briefings concerning Teva’s information security and technology risks, including cybersecurity, and has been briefed on Teva’s information security and risk management programs. Teva’s information security office leads our cybersecurity risk management program.
All of the Audit Committee members have been determined to be independent as defined by SEC and NYSE regulations.
The Board of Directors has determined that, of the current directors on this committee, Gerald M. Lieberman (chair), Murray and Roberto A. Goldberg, Galia Maor and Dan S. SuesskindMignone are “audit committee financial experts” as defined by applicable SEC regulations. Ms. Maor and Mr. Suesskind have each decided not to submit their candidacy for reelection at the Annual Meeting.
Human Resources and Compensation Committee
PubliclyThe Israeli Companies Law requires publicly held Israeli companies are required to appoint a compensation committee. OurAs a NYSE-listed company, Teva’s HR and Compensation Committee includes onlymust be comprised solely of independent directors, as defined by the SEC and NYSE regulations.
The HR and Compensation Committee is responsible for establishing annual and long-term performance goals and objectives for our executive officers, as well as reviewing our compensation philosophy and policies (including our Compensation Policy).
The HR and Compensation Committee is responsible for reviewing plans for the succession of our directors, our chief executive officer and other senior members of executive management.
The HR and Compensation Committee also evaluates the performance of our chief executive officer and other executive officers, makes recommendations to the Board of Directors regarding the compensation of our executive officers and directors, reviews any organizational restructuring pertaining to the roles, responsibilities and selection of executive officers and oversees our labor practices.
All of the HR and Compensation Committee members have been determined to be independent as defined by SEC and NYSE regulations.
Corporate Governance and Nominating Committee
The NYSE Listed Company Manual requires publicly listed companies to appoint a corporate governance / nominating committee composed entirely of independent directors, as defined by NYSE regulations.
The role of our Corporate Governance and Nominating Committee is to (i) identify individuals who are qualified to become directors; (ii) recommend to the Board of Directors director nominees for each annual meeting of shareholders; and (iii) assist the Board of Directors in establishing and reviewing Teva’s statement of corporate governance principles and promoting good corporate governance in Teva.
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 19 | |
Corporate Governance and Director Compensation
All of the committeeCorporate Governance and Nominating Committee members must be, and have been determined to be independent as defined by NYSE regulations.
Finance and Investment Committee
The role of our Finance and Investment Committee is to assist the Board of Directors in fulfilling its responsibilities with respect to our financial and investment strategies and policies, including determining policies on these matters and monitoring implementation. It is also authorized to approve certain financial
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 21
Corporate Governance and Director Compensation
transactions (such as material loans and other financing arrangements), review our financial risk management policies and evaluate the execution, financial results and integration of Teva’s completed acquisitions, as well as various other finance-related matters, including our global tax structure and allocation policies. According to the committee’s charter, at least one of the committee’s members must be qualified as a financial and accounting expert under SEC regulations and/or the Israeli Companies Law.
The Board of Directors has determined that, of the current directors on this committee, Galia Maor, Murray A. Goldberg,Gerald M. Lieberman and Roberto A. Mignone and Dan S. Suesskind(chair) are financial and accounting experts under Israeli law. Ms. Maor and Mr. Suesskind have each decided not
A majority of committee members must be determined to submit their candidacy for reelection at the Annual Meeting.be independent as defined by NYSE regulations.
Compliance Committee
The role of our Compliance Committee is to oversee our: (i) policies and practices for complying with laws, regulations and internal procedures; (ii) policies and practices regarding issues that have the potential to seriously impact our business and reputation; (iii) global public policy positions; (iv) strategy and (iv) social responsibilitygovernance of ESG matters and community outreach.to advise the Board on ESG matters; and (v) implementation of our culture of integrity.
A majority of committee members must be determined to be independent as defined by NYSE regulations. The chairperson of the Audit Committee shall be invited by the committee chairperson to participate in the Compliance Committee, as deemed relevant to the committee’s agenda.
Science and Technology Committee
The Science and Technology Committee oversees our overall strategic direction and investment in research and development and technological and scientific initiatives. As part of this responsibility, it reviews scientific and R&D strategy and priorities, scientific aspects of business development activities and technological trends. It assists the Board of Directors in risk management oversight relating to R&D and our intellectual property, advises on our intellectual property strategy, reviews new technology in which Teva is, or is considering, investing and reviews the efficacy and safety profile of new pharmaceuticals.
All of the committee members must be determined to have scientific, medical or other related expertise. A majority of committee members must be determined to be independent as defined by NYSE regulations.
Code of Business Conduct
Teva has adopted a code of business conduct applicable to its directors, executive officers, and all other employees. A copy of the code is available to every Teva employee on Teva’s internet site, upon request to its human resources department, and to investors and others on Teva’s website at www.tevapharm.com or by contacting Teva’s investor relations department, legal department or the internal auditor. If we make any amendment or grant any waiver to this code that applies to our chief executive officer, chief financial officer, chief accounting officer or controller, or persons performing similar functions, and that relates to an element of the SEC’s “code of ethics” definition, then we will disclose the nature of the amendment or waiver on Teva’s website. The Board of Directors has approved a whistleblower policy which functions in coordination with Teva’s code of business conduct and provides an anonymous means for employees and others to communicate with various bodiesdepartments of Teva, including the Audit Committee. Teva has also implemented a training program for new and existing employees concerning the code of business conduct and whistleblower policy.
| 20 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Corporate Governance and Director Compensation
Principles of Corporate Governance
We have adopted a set of corporate governance principles, which is available on our website at www.tevapharm.com. We place great emphasis on maintaining high standards of corporate governance and continuously evaluate and seek to improve our governance standards. These efforts are expressed in
22 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Corporate Governance and Director Compensation
our corporate governance principles, our committee charters and the policies of our Board of Directors. Teva is in compliance with all corporate governance standards currently applicable to Teva under Israeli and U.S. laws, SEC regulations and NYSE listing standards.
Insider Trading Policy
Our directors, executive officers and employees, as well as their immediate family members, persons living in their home and entities controlled by any of the foregoing persons are subject to Teva’s insider trading policy (the “Policy”). The Policy prohibits insider trading and certain speculative transactions (including short sales, buying put and selling call options and other hedging or derivative transactions in Teva’s securities), and establishes a regular blackout period schedule during which directors, executive officers and certain employees may not trade in Teva’s securities. In addition, the Policy establishespre-clearance procedures that directors and executive officers must observe prior to effecting any transaction in Teva’s securities. The Policy applies not only to Teva’s ADSs and ordinary shares, but also to its debt securities and other securities for which Teva securities serve as underlying assets.
Board Evaluation Process
Our Board of Directors is committed to continuous improvement and recognizes the fundamental role a robust Board of Directors and committee evaluation process play in ensuring that our Board of Directors maintains optimal composition and functions effectively.
TheIn the annual self-evaluation process, the members of the Board of Directors conduct a confidential oral assessment of the performance, risk oversight and composition of the Board and any committees of which he or she is a member with the Company Secretary. As part of the evaluation process, the Board of Directors, in conjunction with the Corporate Governance and Nominating Committee, conducts an annual self-evaluationreviews the effectiveness and overall composition, including director tenure, board leadership structure, diversity and skill sets, of its effectivenessthe Board of Directors to ensure the Board of Directors serves the best interests of shareholders and positions the company for future success. The results of the oral assessments are then summarized and communicated back to each committee, committee chair and the entire Board of Directors. After the evaluations, each committee, committee chair and the entire Board of Directors and management work to improve upon any issues presented during the evaluation process and to identify opportunities where an enhancement or change in practicesthat may lead to further improvement. The Corporate Governance and Nominating Committee also uses this process to assess and determine the characteristics and skills required of prospective candidates for election to the Board of Directors.
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 21 | |
In late 2020 and early 2021, the Board conducted discussions with our shareholders as part of our ongoing commitment to strengthen our corporate governance and to gather input from our stakeholders, which we believe enables us to better understand their perspectives. During this time, we contacted shareholders representing approximately 50% of our outstanding shares. Our Chairman of the Board, the Chair of our HR and Compensation Committee and another member of the Board who presented on ESG matters participated in discussions with shareholders representing approximately 22% of our outstanding shares. In addition, we engaged with the research teams at proxy advisory firms Institutional Shareholder Services Inc. and Glass Lewis & Co.
The discussions covered a broad array of matters, including:
| ∎ | the COVID-19 pandemic and its impact on our human capital; |
| ∎ | our ESG materiality assessment, our enhanced ESG transparency and the resulting improvements in ESG ranking indices; |
| ∎ | inclusion and diversity at Teva; |
| ∎ | executive succession planning; and |
| ∎ | changes to our executive compensation program over recent years. |
Feedback from our shareholders was shared and discussed with the HR and Compensation Committee, the Corporate Governance and Nominating Committee and the Board.
The Board and management continue to engage regularly in dialogue with many of the Company’s largest shareholders, and the HR and Compensation Committee will continue to consider shareholder feedback and the results of the advisory vote on executive compensation in connection with its determinations of executive compensation. Through our shareholder outreach, we have established important feedback channels that provide a valuable way to receive ongoing input from our shareholders.
See also “Executive Compensation—Compensation Discussion & Analysis—2020 Say-on-Pay Vote and Shareholder Engagement.”
Our employees are the heart of our company. We seek to make Teva an inclusive, diverse and safe workplace, with meaningful compensation, benefits and wellness programs, and offering training and leadership development programs that foster career growth.
Our HR and Compensation Committee, Compliance Committee and Board play key roles in overseeing human capital management at Teva and devote time throughout the year to its strategy and execution.
The Board and executive management view inclusion and diversity as essential to our ability to innovate and grow our business. It is our desire to create and sustain an inclusive and diverse work environment.
| 22 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Human Capital Management
As of December 31, 2020, Teva’s work force consisted of 40,216 employees. We have employees in 60 countries around the world, representing a wide range of nationalities. Employees identifying as female represent 45% of our global employee population, 47% of managers and 23% of top executives as of December 31, 2020.
We seek to underscore our inclusive and diverse culture through employee resource groups and training, among other things. For instance, in the U.S., the Teva Inclusion Network is made up of nine employee resource groups, which have a key role in creating a culture of inclusion and bring together employees with shared characteristics and life experiences to foster opportunities for networking, mentoring, collaboration, community outreach, career development, leadership training and cultural exchanges.
Since the start of the COVID-19 pandemic, we have operated conscientiously, focusing on the health, safety and well-being of our employees as a top priority. We have reduced the number of employees in our facilities to enable social distancing by introducing virtual solutions and flexible work arrangements. We adhere to PPE and hygiene instructions to protect our people, their families and the communities where we operate and live.
We enhanced our well-being programs, equipped our managers with information and tools on effective management in times of disruption, and provided employees with online resources to address the challenges of working remotely.
We have been monitoring employee morale during this time in many ways, including by conducting our annual employee survey. Results of the 2020 survey show that employee engagement levels are high. Employees are feeling connected with Teva’s mission and values. They feel pride in Teva’s positive impact on society and have trust in Teva’s future.
For further information, see “Item 1—Business— Human Capital Management” in our Annual Report on Form 10-K for the year ended December 31, 2020.
Environmental, Social and Governance
In 2020, as the world confronted some of the most complex challenges of our time, Teva reevaluated its ESG strategy with the support of its leadership and Board of Directors. Our new strategy views ESG as core to our business and reflects how we address environmental and social issues, while also bringing value to Teva.
Our 2020 materiality assessment identified the ESG topics that we believe matter most to our stakeholders and our business. These topics are the basis of our ESG efforts and complement the areas disclosed in our Annual Report on Form 10-K for the year ended December 31, 2020, together reflecting our financial and non-financial performance. We report annually on how we manage these topics and our progress towards achieving them.
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 23 | |
Environmental, Social and Governance
Of these material topics, we identified ten where we believe Teva can have the greatest impact and which have the greatest impact on our business. These include access to health and medicines, inclusion and diversity, ethics and climate action and resilience. We focus additional resources on these areas, appointing task forces, working groups and committees to set goals and ensure progress.
ESG at Teva
For the health of our patients, our world and our business
Environmental
With 61 manufacturing sites around the world, and a growing list of issues impacting our planet, we have a responsibility to lessen our impact on the environment. This year, Teva set new long-term environmental targets to help advance climate action and resilience, responsible use of natural resources through improved management and reductions in emissions, effluents and waste.
Social
Advancing access to medicines is imperative to our business. We are focused on becoming a committed global health partner addressing unmet needs, increasing the reach of affordable medicines and expanding our generic and innovative medicines portfolio. We aspire to achieve this as a donor, convener and access innovator. Our efforts to create quality, affordable medicines for more people around the world are governed by our Access to Medicines working group and guided by Teva’s Position on Access to Medicines. In 2020, we received marketing authorizations for 800 generic medicines and 100 specialty medicines. We engage partners to advance access initiatives, help reduce healthcare costs and create cost-effective solutions, respond to drug shortages and participate in global health tenders in an effort to make treatments easily accessible and affordable for all.
Governance
As part of our culture of compliance, Teva promotes ethical behavior across our business and expects every employee to comply with relevant laws, regulations and Teva policies. Centered on our values, Teva’s Code of Conduct outlines our policies to uphold high ethical standards and conduct our business with integrity. Teva’s Policy on the Prevention of Corruption is designed to prevent corruption and to ensure Teva complies with applicable laws and regulations.
| 24 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Environmental, Social and Governance
For Teva, upholding a culture of compliance means an environment where all employees and management have a comprehensive understanding of rules, regulations, policies, procedures, best practices and behaviors, as well as accountability for the risks and potential consequences associated with non-compliance. We encourage rigorous, open debate that leads to ethical business decisions. A robust culture of compliance encompasses all aspects of Teva’s global operations—from research and development to sourcing APIs.
ESG Governance
Teva’s Board of Directors oversees ESG activities and provides strategic guidance and direction, receiving updates from its committees on their respective ESG-related activities. The Compliance Committee reviews emerging best practices, trends and key issues related to ESG, oversees our ESG strategy and governance and reports to the Board on ESG matters. In November 2020, the Board of Directors held a dedicated session on ESG and appointed a Board representative to serve as a voice on Teva’s ESG progress in our engagement with shareholders. Our executive management approves all global commitments and targets and reviews Teva’s annual ESG Progress Report. Teva’s dedicated ESG team is responsible for strategy implementation, coordination with relevant internal functions and local markets and engagement with various external stakeholders, as well as reporting, disclosures and communications. Cross-functional task forces, working groups and committees meet quarterly or on an ad-hoc basis to manage individual priority ESG topics. They are responsible for goal setting and coordination with relevant business units to ensure progress and accountability.
Teva 2020 ESG Performance
We continue to enhance ESG transparency, which has already resulted in steady improvements in ESG ranking indices, as evidenced by the chart below:
For more details on Teva’s ESG performance, please see our 2020 ESG Progress Report (expected to be published in May 2021).
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 25 | |
The following table sets forth information regarding our executive officers as of April 25, 2018:10, 2021:
Name
| Age
| Executive
| Position
| |||||||||
Kåre Schultz
|
| 56
|
|
| 2017
|
| President and Chief Executive Officer
| |||||
Iris Beck-Codner
|
| 52
|
|
| 2014
|
| Executive Vice President, Global Brand and Communications
| |||||
Richard Daniell
|
| 51
|
|
| 2017
|
| Executive Vice President, European Commercial
| |||||
Sven Dethlefs
|
| 49
|
|
| 2017
|
| Executive Vice President, Global Marketing & Portfolio
| |||||
Dr. Hafrun Fridriksdottir
|
| 56
|
|
| 2017
|
| Executive Vice President, Global R&D
| |||||
Michael McClellan
|
| 48
|
|
| 2017
|
| Executive Vice President, Chief Financial Officer
| |||||
Gianfranco Nazzi
|
| 49
|
|
| 2017
|
| Executive Vice President, Growth Markets Commercial
| |||||
Dr. Carlo de Notaristefani
|
| 61
|
|
| 2012
|
| Executive Vice President, Global Operations
| |||||
Brendan O’Grady
|
| 51
|
|
| 2017
|
| Executive Vice President, North America Commercial
| |||||
Mark Sabag
|
| 48
|
|
| 2013
|
| Executive Vice President, Global Human Resources
| |||||
David M. Stark
|
| 49
|
|
| 2016
|
| Executive Vice President, Chief Legal Officer
| |||||
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 23
Corporate Governance and Director Compensation
Effective November 1, 2017, Kåre Schultz joined Teva as President and CEO and was also appointed to the Board of Directors. He succeeded Dr. Yitzhak Peterburg, who served as Interim President and Chief Executive Officer from February to October 31, 2017.
On November 27, 2017, Michael McClellan was appointed Executive Vice President, Chief Financial Officer, after serving as Interim Chief Financial Officer since July 1, 2017. He succeeded Eyal Desheh who served as Group Executive Vice President, Chief Financial Officer since 2008.
In November 2017, we announced a new organizational structure and leadership changes to enable strategic alignment across our portfolios, regions and functions. In December 2017, we announced a comprehensive restructuring plan intended to significantly reduce our cost base, unify and simplify our organization and improve business performance, profitability, cash flow generation and productivity. As a result of these changes, Dr. Michael Hayden, Dr. Rob Koremans and Dipankar Bhattacharjee stepped down from their executive roles at Teva on November 27, 2017.
Name | Age | Executive Officer Since | Position | |||||||||
Kåre Schultz | 59 | 2017 | President and Chief Executive Officer | |||||||||
Richard Daniell | 54 | 2017 | Executive Vice President, European Commercial | |||||||||
Sven Dethlefs | 52 | 2017 | Executive Vice President, Global Marketing & Portfolio and International Markets Commercial | |||||||||
Eric Drapé | 59 | 2019 | Executive Vice President, Global Operations | |||||||||
Dr. Hafrun Fridriksdottir | 59 | 2017 | Executive Vice President, Global R&D | |||||||||
Eli Kalif | 48 | 2019 | Executive Vice President, Chief Financial Officer | |||||||||
Brendan O’Grady | 54 | 2017 | Executive Vice President, North America Commercial | |||||||||
Mark Sabag | 51 | 2013 | Executive Vice President, Chief Human Resources Officer and Global Communications and Brand | |||||||||
David M. Stark | 52 | 2016 | Executive Vice President, Chief Legal Officer | |||||||||
Kåre Schultz
President and Chief Executive Officer | The biography of Kåre Schultz, our President and Chief Executive Officer, and one of our directors, appears under “—Directors” above. | |
| ||
Richard Daniell
Executive Vice President, European Commercial | Mr. Daniell was appointed Executive Vice President, European Commercial in | |
| 26 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Executive Officers
Sven Dethlefs
Executive Vice President, Global Marketing & Portfolio and International Markets Commercial | Mr. Dethlefs was appointed Executive Vice President, Global Marketing & Portfolio and International Markets Commercial in | |
Eric Drapé Executive Vice President, Global Operations | Mr. Drapé was appointed Executive Vice President, Global Operations in 2019. From 2015 to 2019, he served as Teva’s Executive Vice President and Chief Quality Officer. From 2014 to 2017 he also served as head of Teva’s Biologics Operations, and from 2014 to 2015 he served as Senior Vice President, Technical Operations Steriles, Respiratory and Biologics at Teva. Prior to joining Teva, Mr. Drapé served as Executive Vice President, Technical Operations of Ipsen Pharma and in several leading positions at Novo Nordisk. Mr. Drapé holds a Doctorate degree in Pharmacy and a DESS in Analytical Control of Drugs from the Université Paris XI. He also received his Executive MBA from the Scandinavian International Management Institute in Copenhagen. | |
Dr. Hafrun Fridriksdottir
Executive Vice President, Global R&D | Dr. Fridriksdottir | |
24 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Corporate Governance and Director Compensation
| President of Global Generics R&D in Allergan | ||
Eli Kalif
Executive Vice President, Chief Financial Officer | Mr. | |
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 27 | |
Executive Officers
| ||
| ||
Brendan O’Grady
Executive Vice President, North America Commercial | Mr. O’Grady was appointed Executive Vice President, North America Commercial in | |
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 25
Corporate Governance and Director Compensation
Mark Sabag
Executive Vice President, Officer and Global Communications and Brand | Mr. Sabag | ||
David M. Stark
Executive Vice President, Chief Legal Officer | Mr. Stark | ||
26 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
| 28 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
COMPENSATION DISCUSSION AND ANALYSIS
This compensation discussion and analysis (“CD&A”) describes the philosophy, objectives, process, components and additional aspects of our 20172020 executive compensation program. This CD&A is intended to be read in conjunction with the tables that immediately follow this section, which provide further historical compensation information for the following named executive officers (“NEOs”):
| Position
| |
Kåre Schultz
|
President and Chief Executive Officer (“CEO”)
| |
|
Executive Vice President, Chief Financial Officer (“CFO”)
| |
Dr. |
| |
|
Executive Vice President, Global
| |
| Executive Vice President, North America Commercial | |
Eric Drapé
|
Executive Vice President, Global | |
| ||
|
| |
|
| |
|
| |
|
| |
|
| |
Quick CD&A Reference Guide
|
Page 29
| |
II. Compensation Philosophy and Objectives
|
| |
III. Compensation Determination Process
|
| |
IV. Components of Our Compensation Program
|
| |
V. Additional Compensation Policies and Practices
|
|
I. Executive SummaryBUSINESS AND COMPENSATION OVERVIEW
OverviewCompensation Objectives
The core objectives of our executive compensation programs are to (i) link pay to performance over both the short- and long-term; (ii) align executive officers’ interests with those of Teva and its shareholders over the long-term, generally by including grants of the Company’s equity as a significant component in our executive compensation program; (iii) encourage balanced risk management; and (iv) provide a competitive compensation package that motivates executive officers. Consistent with these objectives, our compensation plans are designed to reward our executive officers for generating performance that achieves Company, business and individual goals, and for increasing shareholder returns. When we do not achieve Company, business and individual goals, our executive officers’ compensation reflects that performance.
2017 was a year of transition for Teva. Both our generic medicines business and our specialty medicines business faced significant challenges. In order to position Teva for the future,accomplish our key corporate objectives, we made changesmust attract, motivate and retain highly skilled and experienced people to execute our leadership structure. Ourcorporate strategy and lead our team. To that end, our executive officer compensation program is designed to support our strategy, especially during this period of transition.to:
| (i) | link pay to performance; |
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 27
Executive Compensation
| (ii) | align executive officers’ interests with those of Teva and its shareholders over the long term; |
| (iii) | provide competitive compensation to attract and retain talent; and |
| (iv) | encourage performance without excessive risk. |
20172020 Select Business Highlights
In 2020, despite challenges due to COVID-19, we made meaningful progress in executing on our strategic plan and delivered results within our original financial guidance, as detailed below.
|
| ∎ | We successfully navigated our operations through the COVID-19 pandemic with minimal impact on our supply chain, research & development programs and product launches, while focusing on the health, safety and well-being of our employees as a top priority. |
| Teva | 29 | |
Executive Compensation
| ∎ | Sales of AUSTEDO for Huntington’s disease and tardive dyskinesia continue to grow, with $638 million in global revenues in 2020, an increase of 65% compared to 2019. AUSTEDO was |
launched in China in early 2021. |
| ∎ | Sales of AJOVY for the preventive treatment of migraines in adults continued to grow, bolstered by the launch of our |
| ∎ | After its launch in November |
| ∎ | We launched the first generic versions of HIV-1 treatments Truvada and |
| ∎ | We |
| ∎ | We |
| ∎ | We renewed our Environmental, Social, and Governance (“ESG”) strategy, published our annual ESG Progress Report, and in January |
| ∎ | In January 2020, we |
| ∎ | We reduced our net debt by an additional $1.2 billion to a total of $23.7 billion at the |
28 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Executive Compensation
Financial Results
|
| ∎ | Revenues in 2020 were |
| ∎ | Operating loss was $3,572 million in 2020, compared to |
| ∎ | As of December 31, 2020, our debt was $25,919 million, compared to |
| ∎ | GAAP Loss Per Share was |
| ∎ | During 2020, we generated free cash flow of $2,110 million, which was |
| 30 | ||
Executive Compensation
Ensuring Business Continuity and Achieving Performance Goals Despite COVID-19
As a leading global pharmaceutical company, Teva provides essential medicines to millions of patients around the world every day. The COVID-19 pandemic has negatively impacted the global economy, disrupted global supply chains and created significant volatility and disruption of financial markets. Despite these challenges, in 2020, we did not experience significant impacts or delays from the COVID-19 pandemic on our business operations and we delivered financial results in line with our original guidance as communicated in February 2020. We remain focused on the health and well-being of our employees and on our responsibility to continue to provide our medicines to the nearly 200 million patients who depend on us every day.
In 2019, we had essentially concluded our restructuring plan, steadied our earnings performance and begun bringing new specialty products to market around the world. In 2020, we began transitioning to a growth orientation, with several product launches and growth in sales and market share of recently launched products.
Consistent with this strategic shift, the HR and Compensation Committee and the Board shifted the focus of incentives by incorporating Net Revenue into our long-term incentive equity metrics. Non-GAAP EPS and Free Cash Flow remained the financial performance metrics under our annual incentive plan. Even amidst the headwinds and operational impacts of the global pandemic, our CEO and our executive officers led the Company in successfully achieving 101% of the 2020 non-GAAP EPS goal, which reflected an increase of 7% over 2019 actual performance, and achieving 106% of the 2020 Free Cash Flow goal, which reflected an increase of 3% over 2019 actual performance. No adjustments were made to these goals due to the global pandemic.
See “—Key Aspects of 2020 Executive Compensation—3. 2020 Targets Increased and Rigorous; Performance Achievement under Annual and Long-Term Programs Aligned with Targets; No Adjustments Due to COVID-19” below for additional information on the target and achievement levels of performance for these metrics.
Key Aspects of 2020 Executive Compensation
| 1. | 2020 CEO Compensation: Majority Performance-Based (Total and Long-Term Incentive Equity) |
Approximately 86% of our CEO’s 2020 compensation was variable and at-risk, with the substantial majority being performance-based. In addition, 70% of the long-term incentive equity grants were in the form of performance share units (“PSUs”). The PSUs are subject to three-year performance metrics tied to our key goals and there is a relative Total Shareholder Return (“TSR”) modifier (+/- 20%). The PSUs will vest, if at all, at the end of the performance period. The other 30% of long-term incentive equity grants were in the form of time-based restricted share units (“RSUs”), which vest over four years. In 2020, our shareholders approved an increase in the target grant date fair value of the CEO’s long-term incentive equity award by $4 million in connection with an amendment to his employment agreement, in order to bring his target total direct compensation in line with the market median range. The increase, which took the form of performance-based PSUs, raised the performance-based proportion of long-term incentive equity to 70%, as described above, and the performance-based portion of total direct compensation to 66%. There was no increase to the CEO’s annual base salary or target annual incentive as compared to 2019.
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | ||
Components of Compensation and Target Pay Mix
The Compensation Committee, Board and shareholders selected the components of compensation set forth below to achieve our stated executive officer compensation objectives. The majority of the compensation of each executive officer is variable and at risk. We consider compensation to be “at risk” if it is subject to performance-based payment or vesting conditions or if its value depends on share price appreciation.
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 29
Executive Compensation
2020 CEO Target Total Direct Compensation
Executive
| Principal
| Base Salary
| Target
| Target
| Target
| Total (*)
| ||||||
Kåre Schultz |
CEO | $2,000,000 | $2,800,000 | $7,000,000 | $3,000,000 | $14,800,000 | ||||||
% of Total | 14% | 19% | 47% | 20% | 100% | |||||||
% of Long-Term Incentive | 70% | 30% | ||||||||||
| (*) | Equity values have been rounded to the nearest $10,000. |
| 2. | 2020 Long-Term Incentives: 70% Performance-Based Equity for CEO, 50% for all other NEOs; High Threshold Performance Level; Three-Year Performance Goal |
Similar to 2019, 50% of our executive officers generally consiststhe value of annual base salary, annual cash incentives and annual equity-based compensation. As required by the Israeli Companies Law, we have adopted the Compensation Policy, which is presentedtarget equity grant for shareholder approval at least once every three years. Under the Compensation Policy, our target range for the pay mix between the annual base salary, annual cash incentives and annual equity-based compensation of our executive officers is subject to performance-based vesting conditions in the form of PSUs (excluding the CEO, whose target equity grant is 70% performance-based, as follows:described in Item 1 above), and the other 50% is in the form of time-based RSUs. This enhances the strong link between pay and performance for our NEOs and the alignment of the interests of the NEOs with those of Teva and its shareholders.
A minimum of 85% of target performance must be achieved in order to earn any PSUs for a particular metric, which is a rigorous and challenging level of achievement that must be met before any PSUs are earned. In addition, the HR and Compensation Committee and the Board established three-year goals for the performance period.
| 3. | 2020 Targets Increased and Rigorous; Performance Achievement under Annual and Long-Term Programs Aligned with Targets; No Adjustments Due to COVID-19 |
No Adjustments Due to COVID-19. The HR and Compensation Committee and the Board did not make any adjustments to the targets and did not exercise any discretion to make adjustments to our annual or long-term incentive plan goals due to COVID-19, demonstrating our strong performance relative to those goals despite challenging circumstances.
Annual Cash Incentives. At the beginning of 2020, we established annual cash incentive plan targets for Non-GAAP EPS and Free Cash Flow that were aligned to the ranges of our outlook as communicated to investors in February 2020. These goals were considered rigorous, aggressive and challenging, attainable only with strong performance, and took into account the relevant opportunities and risks, including the significant continuing headwinds we were facing.
The 2020 Non-GAAP EPS goal was set at $2.55, 6% higher than our actual 2019 performance and 2% higher than the 2019 goal, and the Free Cash Flow goal remained the same as 2019 at $2.0 billion, based on the assumptions communicated to investors in February 2020, including:
| ∎ | Anticipated continued decline in COPAXONE revenue from $1.5 billion in 2019 to approximately $1.2 billion in 2020 due to an expected increase in generic competition; |
| ∎ | Anticipated continued increase of AUSTEDO revenue in the U.S. from $412 million in 2019 to $650 million in 2020; and |
| ∎ | Anticipated continued increase of global AJOVY revenue from $96 million in 2019 to $250 million in 2020. |
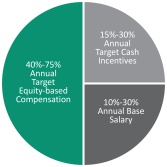
The target ranges express the optimal pay mix in the event all performance measures are achieved at target levels, and assume all compensation elements are granted with respect to a given calendar year. Performance that is lower than target levels or exceeds target levels in any given calendar year may result in a payout in different percentages than those described above.
The target pay mix supports the core principles of our executive officer compensation philosophy of compensating for performance and aligning executive officers’ interests with those of Teva and its shareholders, by emphasizing short- and long-term incentives that fall within the ranges noted above.
Corporate Governance Practices
As partIn spite of the efforts ofchallenges we faced in 2020, we achieved very solid results. For the 2020 annual incentive plan, the HR and Compensation Committee to ensureand the Board determined that our compensation program, which includes our policies and practices, aligns our executive officers’ interests with those of Teva and its shareholders, the Compensation Committee assesses the effectiveness of our compensation program periodically, and reviews risk mitigation and governance matters. We do this by maintaining the following best practices:Company’s
| 32 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |||||
Executive Compensation
achievement was 101% of our 2020 Non-GAAP EPS target, and 106% of our 2020 Free Cash Flow target. Based on achievement of these corporate objectives, along with determination of the NEOs’ individual performance achievement (which represented 25% of the target total opportunity), the HR and Compensation Committee and the Board approved an annual incentive plan payout of 109% of target for the CEO and between 115% and 128% of target for the other NEOs.
Long-Term Incentives. For 2018-2020 PSUs, the HR and Compensation Committee and the Board determined that the achievement for the combined three-year performance period was 106% of the target for Non-GAAP EPS, resulting in an earning percentage of 131%, and achievement of 117% of the target for Free Cash Flow, resulting in an earning percentage of 185% (2018 was the last year before we changed to different metrics for the PSUs; in 2019 the metrics were Net Debt and Non-GAAP Operating Profit with a Relative TSR Modifier and in 2020 the metrics were Net Revenue and Non-GAAP Operating Profit with a Relative TSR Modifier). The average of these earning percentages, 158%, was then subject to a relative TSR modifier, which adjusted the earning percentage downward by 20%, resulting in a modified earning percentage of 126% of the target number of PSUs.
Realizable Pay Demonstrates Pay for Performance Alignment
Realizable pay reflects the actual value of annual incentives and equity awards received or to be received by our NEOs, and fluctuates with performance and with increases or decreases in share price. For this reason, contrasting target pay with realizable pay provides a meaningful demonstration of pay for performance alignment.
When the Company does not meet performance targets and/or the share price decreases, an executive’s realizable pay is affected. The following charts demonstrate the relationship between the target and realizable pay values, in each of the past three years, of our NEOs’: (1) annual incentive, and (2) annual equity grants, including PSUs, RSUs, and stock options, as applicable, following the conclusion of the applicable three-year PSU performance period (December 31) and RSU and option values from the same annual grant as of that date. The equity grants to the NEOs in 2016 included PSUs and stock options and in 2017 and 2018 included PSUs, RSUs and stock options. The realizable value of this equity has been calculated by multiplying the number of earned shares for each PSU grant and the number of vested and unvested RSUs (for the 2017 and 2018 grants) by the stock price per share on the last trading day of the relevant performance period, and by determining the intrinsic (or “in the money”) value of vested and unvested stock options on these dates, as applicable.
With respect to PSUs, for 2016 grants, the Company’s three-year performance produced an earning percentage of 75%, but because of the decrease in the share price, the realizable value of such PSUs represented just 22% of the target value.
For NEOs that received a grant in February 2017, the Company’s three-year performance produced an earning percentage of 100%, but because of the decrease in the share price, the realizable value of such PSUs represented just 35% of the target value, and the realizable value of RSUs represented just 31% of the grant value. Per the terms of Mr. Schultz’s employment agreement, he received a grant of PSUs, RSUs, and stock options upon commencing employment in November 2017. The decline in the share price that had taken place before his grant date resulted in a lower grant date fair value per unit, which led the CEO’s realizable value of such PSUs to represent 104% of the target value and the realizable value of RSUs to represent 94% of the grant value.
For 2018 grants, the Company’s three-year performance produced an earning percentage of 126%, but because of the decrease in the share price, the realizable value of such PSUs represented just 72% of the target value and the realizable value of RSUs represented just 52% of the grant value.
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 33 | |
Executive Compensation
With respect to stock options granted in 2016, 2017, and 2018, because of the decrease in the share price, no stock options granted had any intrinsic value at the conclusion of the respective PSU performance periods.
Average NEO Annual Cash Incentive Payout as % of Target | Average NEO Annual Equity Grant (PSUs, RSUs, Options) Realizable Value as % of Target Value | |
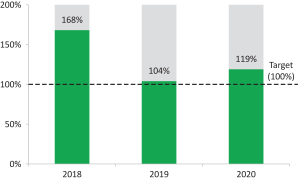 | 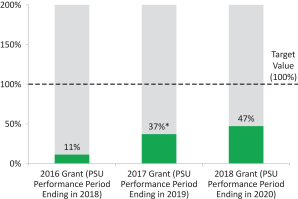 | |
* The average NEO 2017 grant realizable value is 22% excluding the CEO’s annual equity award granted in November 2017, which had a lower fair value per unit when it was granted. |
No Earned Shares for CEO 3-Year Sign-On PSU Grant.In 2017, in connection with the recruitment and inducing of Kåre Schultz to serve as CEO of Teva, the Company granted three-year and five-year sign-on PSUs to Mr. Schultz. The three-year PSUs had a target grant date fair value of $7.5 million (as determined based on the closing price of Teva’s shares on the date prior to the announcement of Mr. Schultz’s hire), to be earned based on the achievement of performance goals related to the absolute increase in the price of Teva’s shares over the three-year period following the commencement date of his employment. The performance goals ranged from a 16% increase to a 158% increase for the three-year performance period, and generally would have vested on the third, fourth and fifth anniversaries of the commencement date. At the end of the three-year performance period, the stock price had not achieved the threshold level. Accordingly, no shares were earned in respect of the three-year sign-on PSUs, which underscores the substantial pay for performance orientation of our CEO’s compensation. The five-year PSU performance period will end in 2022.
2020 Say-on-Pay Vote and Shareholder Engagement
At the 2020 annual meeting of shareholders, our shareholders approved, on an advisory basis, in our Say-on-Pay proposal, the compensation of our NEOs, with approximately 79% of the votes cast on the matter “For” such approval.
In late 2020 and early 2021, the Board conducted discussions with shareholders, as part of our ongoing commitment to strong corporate governance and continuous dialogue with our stakeholders, which we believe enables us to better understand their perspectives.
During this time, we contacted shareholders representing approximately 50% of our outstanding shares. Our Chairman of the Board, the Chair of our HR and Compensation Committee and another member of the Board who presented on ESG matters participated in discussions with shareholders representing approximately 22% of our outstanding shares. In addition, we engaged with the research teams at proxy advisory firms Institutional Shareholder Services Inc. and Glass Lewis & Co.
| 34 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Executive Compensation
The discussions covered a broad array of matters as presented in the table below.
| The Chair of our HR and Compensation Committee raised the topic of our executive compensation program. Shareholder commentary on the subject was limited, and ultimately, there were no common themes. (Given the opportunity to determine the topics of discussion, most shareholders focused on the other topics set forth below.) Following engagement with shareholders over the past few years, the HR and Compensation Committee has taken the following actions:
∎ Increased the proportion of long-term incentive equity grants that are performance-based from 50% to 70% for the CEO (2020) and from 33% to 50% for all other NEOs (2019); ∎ Introduced a ∎ Enhanced disclosure to show the threshold, target and maximum performance levels and payout opportunities for the annual cash incentive plan and for PSUs, the performance period of which has ended (2018); ∎ Eliminated overlapping metrics in short- and long-term performance-based awards (2019); ∎ Updated peer group criteria by reducing the revenue range to $10-$40 billion from $10-$70 billion, which resulted in the removal of five of the largest companies by revenue, to reflect current organizational status (2019); ∎ Enhanced stock ownership guidelines by increasing the ownership guideline for the CEO to 6x base salary (from 4x) and for the other executive officers to 3x base salary (from 2x), adopted ownership guidelines for members of the Board of Directors equal to 5x the annual cash retainer fee for Board membership (excluding committee fee) and discontinued including unvested PSUs toward satisfaction of the ownership guidelines (2019); and ∎ Requested and obtained shareholder approval to decrease compensation for the Chairman of the Board and revise the compensation for other directors to provide greater alignment with shareholders (2019). | |||||
COVID-19 and its impact on our human capital | Our HR and Compensation Committee, Compliance Committee and Board play key roles in overseeing human capital management at Teva and devote time throughout the year to its strategy and execution.
|
| ||||
We enhanced our well-being programs, equipped our managers with information and tools on effective management in times of disruption, and provided employees with online resources to address the challenges of working remotely.
|
|
|
| |||
|
|
|
have trust in Teva’s future. | |||
15%-30% Annual Target Cash Incentives 40%-75% Annual Target Equity-based Compensation 10%-30% Annual Base Salary
30 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 35 | |
Executive Compensation
| Topics Raised | Teva Response | |
Environmental, Social, and Governance | The Board and the executive management team firmly believe that ESG, or “how” we achieve our goals and underscore non-financial performance, is critical to our long-term sustainability and success. In 2020, Teva conducted a robust materiality assessment with input from internal and external stakeholders to identify the ESG topics most important to our business and where Teva can have the greatest impact. We invested in programs to address global health challenges such as chronic diseases and access to medicines, improved our environmental sustainability, and strengthened controls to ensure ethical conduct. We publish an annual ESG progress report, and in January 2021, externally communicated our ambitious long-term environmental goals. We continue to enhance transparency, which has already resulted in steady improvements in ESG ranking indices. Sustainalytics ranked Teva in the top 10% of pharmaceutical companies and named us an outperformer for environmental performance. Our Dow Jones Sustainability Index performance improved from the 55th to the 70th percentile in our industry over the last year. Our CDP climate change score improved from a B to an A- in the last year, ranking Teva in the top 38% of companies. | |
Inclusion and diversity | The Board and executive management view inclusion and diversity as essential to our ability to innovate and grow our business. It is our desire to create and sustain an inclusive and diverse work environment. Employees identifying as female represent 45% of our global employee population, 47% of managers, 23% of top executives, and 25% of directors as of December 31, 2020. We seek to underscore our inclusive and diverse culture through employee resource groups and training, among other things. | |
Executive succession planning | The HR and Compensation Committee and Board view succession planning as a top priority. Our succession planning covers the CEO as well as our executive management team. The goal of succession planning is to ensure we have the highest caliber talent pool to lead our strategic growth in a collaborative environment. Both internal and external candidates will be considered but we are highly encouraged by our commitment to develop internal talent. The CEO is fully engaged in this process. | |
Feedback from our shareholders was shared and discussed with the HR and Compensation Committee, the Corporate Governance and Nominating Committee and the full Board.
| 36 | Teva Pharmaceutical Industries Ltd. | |||||
Executive Compensation
The Board and management continue to engage regularly in dialogue with many of the Company’s largest shareholders, and the HR and Compensation Committee will continue to consider shareholder feedback and the results of the advisory vote on executive compensation in connection with its determinations of executive compensation as depicted in our annual shareholder engagement cycle below. Through our shareholder outreach, we have established important feedback channels that provide a valuable way to receive ongoing input from our shareholders.
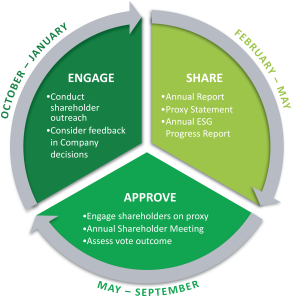
II. COMPENSATION PHILOSOPHY AND OBJECTIVES
Compensation Philosophy
✓
| ∎ |
|
| ∎ | Alignment of executive officers’ interests with those of Teva and its shareholders: In order to promote retention and motivate executive officers to focus on long-term objectives and the performance of Teva’s shares, a significant portion of the compensation packages of our executive officers is granted in the form of equity-based compensation, which creates a direct link between the interests of executive officers and the interests of Teva and its shareholders. By making executive officers shareholders with a personal stake in the value of Teva, we are motivating them to create, and enabling them to share in, Teva’s growth and success, while also fostering an ownership culture among executive officers. |
| ∎ |
| ||||||
|
|
|
| |||||
|
|
|
| |||||
|
|
X
No highly leveraged incentive plans that encourage excessive risk taking
|
|
|
| ||||||
|
| ||||||||
|
| ||||||||
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 37 | ||||||||
Executive Compensation
| ∎ | High standards of corporate governance, compliance and risk management: We are committed to transparent and ethical business practices. Maintaining high standards of corporate governance and legal compliance are key factors in our success. This allows us to create long-term value for our shareholders as well as all of our other stakeholders, including employees, customers, suppliers and, above all, patients worldwide. Compensation is structured in a manner that creates an incentive to deliver high performance (both short- and long-term) while taking into account our compliance and risk management philosophy and avoiding undue pressure on executive officers to take excessive risks, thereby encouraging a balanced and effective risk-taking approach. |
U.S. Domestic Issuer Status
Effective January 1, 2018, we began filing periodic reports and registration statements with the SEC as a U.S. domestic issuer, after we determined that, as of June 30, 2017, we no longer qualified as a foreign private issuerCompensation Policy under SEC rules. As a U.S. domestic issuer, we must now, for the first time, make our SEC filings under the rules applicable to U.S. domestic issuers, and must include certain disclosures that were not previously required, including this Compensation Discussion and Analysis. In addition, the determination and presentation of executive compensation amounts for NEOs contained within this Executive Compensation section follows SEC and applicable accounting requirements, which differ from the determination and presentation methodologies that were permitted by, and that we have used previously in, prior Annual Reports filed on Form20-F and other disclosures and filings. For example, certain elements of compensation in this Executive Compensation section are reported based on the year with respect to which they were granted or earned, not for periods with respect to which they were accrued or expensed for accounting purposes (as was the case in our prior Annual Reports filed on Form20-F). This applies, for example, to amounts in respect of equity compensation in the Summary Compensation Table, which are now calculated based on grant date fair value and not calculated based on compensation expense as accrued for accounting purposes, as reflected in our prior Annual Reports filed on Form20-F.
Compensation-Related Requirements of the Israeli Companies Law
Due to our unique position as an Israeli company with an extensive global footprint, we aim to adopt compensation policies and practices that match those of similar global companies, but we must also comply with applicable Israeli law, including the requirement that Israeli publicly-traded companies adopt a compensation policy which is submitted periodically for shareholder approval and contains certain limits on elements of compensation. Executive compensation decisions must generally be consistent with that policy.
As approved at our 20162019 annual general meeting of shareholders, and as required by the Israeli Companies Law, we have adopted a Compensation Policy regarding the terms of office and employment of our “office holders”“Office Holders” (as defined under the Israeli Companies Law, which includes directors, the CEO, other executive officers and any other managers directly subordinate to the CEO), including cash compensation, equity-based awards, releases from liability, indemnification and insurance, severance, and other benefits (the “Terms of Office and Employment”). Each of our NEOs is (or was, while employed by us) an “office holder”Office Holder within the meaning of the Israeli Companies Law. The Compensation Policy is reviewed from time to time by the HR and Compensation Committee and the Board to ensure its alignment with our compensation philosophy and objectives and to consider its appropriateness for Teva and isTeva. Under the Israeli Companies law, we are required to be broughtsubmit the compensation policy to shareholders at least once every three years to our shareholders for approval.
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 31
Executive Compensation
Pursuant to the Israeli Companies Law, arrangements between Teva and its office holders must generally be consistent with the Compensation Policy. However, under certain circumstances, we may approve an arrangement that is not consistent with the Please see “—Compensation Policy if the arrangement is approved by a majority of our shareholders, provided that (i) the majority includes a majority of the votes cast by shareholders who are present and voting (abstentions are disregarded) who (A) are not controlling shareholders and (B) do not have a personal interest in the matter, or (ii) the votes cast against the arrangement by shareholders who are not controlling shareholders and who do not have a personal interest in the matter who were present and voted constitute two percent or less of the voting power of the Company (a “special majority”). Under certain circumstances, if the Compensation Policy is not approved by the shareholders, the Compensation Committee and the Board may nonetheless approve such policy.
In addition, pursuant to the Israeli Companies Law, the Terms of Office and Employment generally require the approval of the Compensation Committee and the Board. The Terms of Office and Employment as applicable to directors further require the approval of the shareholders by a simple majority. The Terms of Office and Employment with respect to a CEO generally require the approval of the shareholders by the special majority referenced in the immediately preceding paragraph. Pursuant to regulations promulgated under the Israeli Companies Law, shareholder approval is not required with respect to Terms of Office and Employment granted to a director or a CEO for the period following his or her appointment until the next general meeting of shareholders, provided these terms are (i) approved by the Compensation Committee and the Board, (ii) consistent with the Compensation Policy and (iii) on similar or less favorable terms than those of the person’s predecessor. In addition, under certain circumstances, shareholder approval is not required with respect to the Terms of Office and Employment of a candidate for CEO if the Compensation Committee determines that the engagement will be frustrated if the approval is pursued, provided that the terms are consistent with the Compensation Policy. This provision was followed in the recruitment of our President and CEO, Kåre Schultz.
Under certain circumstances, if the Terms of Office and Employment of office holders who are not directors are not approved by the shareholders, where such approval is required, the Compensation Committee and the Board may nonetheless approve such terms. In addition,non-material amendments of the Terms of Office and Employment of office holders who are not directors may be approved by the Compensation Committee only andnon-material amendments of the Terms of Office and Employment of office holders who are not directors and excluding the CEO may be approved by the CEO only, provided such approvals are permitted under the Compensation Policy and consistent therewith. Accordingly, for as long as not otherwise determined by the Compensation Committee and the Board, our President and CEO is currently authorized to approve benefits and perquisites for any other executive officer with respect to any calendar year, provided that it does not exceed the value of such executive officer’s one month base salary.Requirements” below.
Compensation Proposal Results and Shareholder FeedbackGovernance
We pay careful attention to any feedback we receive fromThe HR and Compensation Committee assesses the effectiveness of our shareholders about our executive compensation program. Although we have not been subject to the requirement for a shareholder advisory vote on our executive compensation program(“say-on-pay”) in the past, we have historically received high levels of support from our shareholders on executive compensation matters, including the approval of our Compensation Policy. At our 2017 annual meeting of shareholders, the votes on executive compensation matters received periodically and reviews risk mitigation and governance matters. We do this by maintaining the following levels of support:best practices:
What We Do | ||
Shareholder Engagement | We reach out to shareholders to understand and address their perceptions and concerns regarding our executive compensation program. | |
Shareholder-Approved Compensation Policy | We must generally comply with the provisions of | |
Majority Variable Pay | The majority of total executive compensation is variable and at-risk, and a meaningful percentage is performance-based. | |
Balance Short- and Long-Term Compensation | The allocation of incentives among the annual incentive plan and the long-term incentive plan does not over-emphasize short-term performance at the expense of achieving long-term goals. | |
Combination of Balanced Performance Metrics | We use a diverse set of performance metrics in our incentive plans to ensure that no single measure affects compensation disproportionately. | |
| 38 | ||
32 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Executive Compensation
The Compensation Committee believes these results demonstrate strong shareholder support for our executive compensation program. While we received such support for our compensation proposals at our 2017 annual general meeting, the Compensation Committee continued to work to enhance our executive compensation program to further align with shareholder interests. When making compensation decisions for our executive officers, the Compensation Committee will continue to consider the outcome of votes on compensation-related matters and feedback from shareholders.
II. Compensation Philosophy and Objectives
To remain competitive, we must attract and retain highly talented professionals with the necessary skills and capabilities to promote creativity and manage global operations. Due to our unique position as an Israeli company with an extensive global footprint, we aim to adopt compensation policies and practices that match those of similar global companies, while complying with applicable local laws and policies.
We are also committed to transparent and ethical business practices. Maintaining high standards of corporate governance and legal compliance are key factors in our success. This allows us to create long-term value for our shareholders as well as all of our other stakeholders, including employees, customers, suppliers and, above all, patients worldwide.
Our executive officer compensation philosophy also values the following principles:
Our objectives with respect to executive officer compensation, as summarized below, are designed to: (i) link pay to performance; (ii) align executive officers’ interests with those of Teva and its shareholders over the long-term; (iii) encourage balanced risk management; and (iv) provide a competitive compensation package that motivates our executive officers.
|
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 33
Executive Compensation
What We Do | ||
Equity for Long-Term Incentives | We use PSUs with | |
Independent Compensation Consultant | Our HR and Compensation Committee has engaged an independent compensation consultant to provide information and advice for use in | |
Peer Data | We review compensation benchmark data from peer companies whose industry, revenues, and global footprint share similarities with Teva. | |
Stock Ownership Guidelines | We maintain guidelines for executive officers and directors to maintain meaningful levels of stock ownership. | |
Clawback | We maintain a clawback policy to recoup cash and equity-based incentives paid to | |
Risk Assessment | We conduct an annual risk assessment of our compensation program. | |
Cap Bonus, Equity Grant Fair Values and PSU Payouts | We cap annual cash incentive payouts, annual equity grant date fair values at target, and the number of PSUs that may | |
Double Trigger Change-in-Control Provisions | If there is a change in | |
What We Don’t Do | ||
No Dividends on Unearned Awards | Under our equity plan, we do not pay dividends or dividend equivalents on shares that a participant has not yet earned or that have not vested. | |
No Hedging or Pledging of Company Securities | We | |
No Repricing of Underwater Stock Options | Our equity plan does not permit the repricing of stock options where the strike price exceeds the then-current fair market value without shareholder approval. | |
No Excise Tax Gross-Ups | We do not provide excise tax gross-ups in employment agreements. | |
No Guaranteed Bonuses | We do not provide guaranteed performance bonuses to | |
No Backdating, or | We do not backdate stock options or | |
III. Compensation Determination Process
The Compensation Committee and the Board design the executive compensation program with the intention of accomplishing the goals of linking pay to performance and creating alignment with Teva and its shareholder interests and also retaining and motivating a qualified executive team to provide strategic leadership and business continuity. In determining executive compensation, the Compensation Committee obtains input and advice from independent compensation consultants as applicable and reviews recommendations from our CEO with respect to the performance and compensation of our other executive officers. The Board, upon recommendation from, and following approval of, the Compensation Committee, reviews and approves compensation and performance awards of the CEO and executive officers. The Compensation Committee and the Board consider financial, operational and share price performance to determine appropriate executive compensation parameters.
34 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 39 | |
Executive Compensation
III. COMPENSATION DETERMINATION PROCESS
Key Participants
The roles and responsibilities of all parties involved with the compensation determination process are set forth below:
| Responsibilities | |
Shareholders |
∎ Approve the Compensation Policy as required under the Israeli Companies ∎ Cast advisory vote on proposal(s) regarding executive compensation under U.S. law ∎ Approve any compensation that deviates from the Compensation Policy ∎ Approve compensation of the CEO ∎ Approve compensation of directors ∎ Approve equity plans, material changes to equity plans and share reserve increases ∎ Provide direct feedback and input to Teva and our Board | |
Board of Directors |
∎ Evaluate performance of the CEO and executive officers, including the NEOs ∎ Review and approve (subject to shareholder approval in certain cases): ∎ Equity plans, material changes to equity plans and share reserve increases ∎ ∎ Changes to the Compensation Policy | |
Human Resources and Compensation Committee |
∎ Consider ∎ Review and approve (subject to Board and shareholder approval in certain cases): ∎ ∎ ∎ Achievement of performance-based goals under the annual cash incentive plan and associated with PSUs ∎ Equity plans and awards ∎ The Compensation Policy and its ∎ The CD&A and the compensation tables and accompanying narrative descriptions | |
Independent Compensation Consultant |
∎ ∎ ∎ Selection of peer group companies ∎ ∎ Design of annual cash incentive plan and performance and other equity plans, and awards and grants under each plan ∎ Stock ownership guidelines ∎ Review and provide an independent assessment of the data and materials presented by management to the HR and Compensation Committee ∎ Participate in HR and Compensation Committee meetings as requested | |
CEO |
∎ Evaluate the performance of other executive officers, including the other NEOs, and recommend adjustments to base salaries, annual cash ∎ Develop business goals, which are | |
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 35
| 40 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Executive Compensation
Role of Independent Compensation Consultant
The HR and Compensation Committee has the authority to retain independent compensation consultants to assist it in the performance of its duties and responsibilities without consulting or obtaining the approval of management of the Company. The HR and Compensation Committee has retained Pay GovernanceSemler Brossy Consulting Group LLC (“Semler Brossy”) as its independent compensation consultantconsultant. Semler Brossy reported directly to, provide advice onand was directly accountable to, the compensation of our executive officers. Pay Governance provides no other services to Teva. TheHR and Compensation Committee. While the HR and Compensation Committee hastook into consideration the review and recommendations of this independent advisor when making decisions about the Company’s executive officer and director compensation practices and governance related topics, the HR and Compensation Committee ultimately made its own independent decisions about these matters.
The HR and Compensation Committee assessed the independence of Pay GovernanceSemler Brossy pursuant to the rules of the SEC and the NYSE. In doing so, the HR and Compensation Committee considered the factors set forth by the SEC and NYSE with respect to a compensation consultant’s independence. The HR and Compensation Committee also considered the nature and amount of work performed for the HR and Compensation Committee and the fees paid for those services in relation to the firm’s total revenues. After these reviews, the HR and Compensation Committee concluded that the engagement of this firm does not raise any conflictthere were no conflicts of interest, with Teva or any of its directors or executive officers.and that Semler Brossy was independent pursuant to SEC and NYSE rules.
Compensation Peer Group and Peer Selection Process
AsThe HR and Compensation Committee believes that obtaining relevant market and benchmark data is very important to making determinations about executive compensation. This information provides a partsolid reference point for making decisions and very helpful context even though, relative to other companies, there are differences and unique aspects of setting the Company.
The HR and Compensation Committee takes into consideration the structure and components of, and the amounts paid under, the executive compensation programs of other, comparable peer companies, as derived from public filings and other sources when making decisions about the structure and component mix of our CEOexecutive compensation program. The HR and executive officers, the Compensation Committee also considers broader industry practices and the Board use comparative compensation information fromour competitors for talent.
The HR and Compensation Committee has developed and maintained a relevant peer group of peer companies (the “Peer Group”) as a data point.
The Compensation Committee selectsusing the companies in the Peer Group, with the assistance of Willis Towers Watson, based on primary selection criteria including, but not limited to, the following:following criteria:
| ∎ | Industry:Pharmaceutical sector/ |
| ∎ | Company |
| ∎ | Global presence and geography: Global footprint and breadth, with focus on U.S. and European |
TheRevenues are considered a primary factor in determining the Peer Group, which has been constructed in part, suchso that our revenues are generally in the middle of the revenue range of the Peer Group companies. In general, the HR and Compensation Committee accords less weight to market capitalization due in part to the number of factors that can influence and cause volatility in the spot price of the common stock of a company. The HR and Compensation Committee believes that the Peer Group companies also representare the companies with which we compete for talent. Periodically,
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 41 | |
Executive Compensation
The Peer Group established for setting 2020 compensation consisted of the following companies:
Company | Headquarters | Revenues (1) ($ in millions) | Market Cap (1) ($ in millions) | Employees (1) | ||||||||||
AbbVie, Inc.
|
United States
|
$
|
32,753
|
|
$
|
99,975
|
|
|
30,000
|
| ||||
Allergan Plc
|
Ireland
|
$
|
15,787
|
|
$
|
53,555
|
|
|
16,900
|
| ||||
Amgen, Inc.
|
United States
|
$
|
23,768
|
|
$
|
124,576
|
|
|
21,500
|
| ||||
Astellas Pharma, Inc.
|
Japan
|
$
|
11,785
|
|
$
|
26,712
|
|
|
16,243
|
| ||||
AstraZeneca Plc
|
United Kingdom
|
$
|
21,137
|
|
$
|
116,266
|
|
|
64,400
|
| ||||
Biogen, Inc.
|
United States
|
$
|
12,053
|
|
$
|
41,421
|
|
|
7,800
|
| ||||
Bristol-Myers Squibb Co.
|
United States
|
$
|
22,561
|
|
$
|
78,958
|
|
|
23,300
|
| ||||
Eli Lilly & Co.
|
United States
|
$
|
24,556
|
|
$
|
110,108
|
|
|
38,680
|
| ||||
Gilead Sciences, Inc.
|
United States
|
$
|
22,214
|
|
$
|
82,092
|
|
|
11,000
|
| ||||
GlaxoSmithKline Plc (2)
|
United Kingdom
|
$
|
39,325
|
|
$
|
104,629
|
|
|
95,490
|
| ||||
Merck KGaA
|
Germany
|
$
|
17,017
|
|
$
|
46,709
|
|
|
51,713
|
| ||||
Mylan NV
|
United Kingdom
|
$
|
11,428
|
|
$
|
10,586
|
|
|
35,000
|
| ||||
Novo Nordisk A/S
|
Denmark
|
$
|
17,174
|
|
$
|
126,116
|
|
|
43,202
|
| ||||
Sanofi
|
France
|
$
|
39,529
|
|
$
|
112,263
|
|
|
104,226
|
| ||||
Takeda Pharmaceutical Co., Ltd.
|
Japan
|
$
|
18,920
|
|
$
|
54,477
|
|
|
49,578
|
| ||||
|
| Revenues ($ in millions) | Market Cap ($ in millions) | Employees | ||||||||||||
Teva Pharmaceutical Industries Ltd. Percentile Rank
|
40th
|
0th
|
60th
| ||||||||||||
Median
|
$21,137
|
$82,092
|
35,000
| ||||||||||||
| (1) | Source (revenue, market capitalization and employee number information): Dow Jones (September 2019). |
| (2) | New peer company added for 2020. |
The HR and Compensation Committee reassesses the companies withinperiodically reviews the Peer Group and makes changes as appropriate, considering changes to ensure that the companies inincluded continue to meet the primary selection criteria outlined above. The HR and Compensation Committee revised the Peer Group such as mergersfor 2020 by adding GlaxoSmithKline Plc since it met the Peer Group criteria. In addition, Celgene Corp. and acquisitionsShire plc were removed due to being acquired and changes in our business.Bayer AG was removed because it no longer met the Peer Group criteria.
The HR and Compensation Committee and the Board consider data from the Peer Group companies in the Peer Group to review the componentsreviewing market pay levels, allocations and practices. Other factors considered when setting the total compensation of our CEO and executive officers relative to their counterparts at Peer Group companies, while also taking into considerationinclude sustained performance, criticality of contributions to Teva, and the executive officer’s role, skills, experience and development. The Compensation CommitteeHR and the Board use Peer Group data as a reference point for measurement, but Peer Group data is just one of several factors considered. The Compensation Committee retains discretion in determining the nature and extent of the use of Peer Group data.
36 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Executive Compensation
The Peer Group established for setting 2017 compensation consisted of the following companies:
| ||
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
|
|
Revenues ($ in millions) | Market Cap ($ in millions) | Employees | ||||
Teva Pharmaceutical Industries Ltd. Percentile Rank
|
47th
|
16th
|
58th
| |||
Median
|
22,859
|
98,638
|
41,275
|
Internal Considerations
Internal fairness: As a global company with complex operations worldwide. The HR and with many of our executive officers and a majority of our employees located outside of Israel, we position our executive officer compensation on a competitive scale commensurate with each executive officer’s role and responsibilities. Due to the large variations in customary pay levels, compensation practices and mandatory compensation requirements among the jurisdictions in which executive officers and employees are located, the Compensation Committee and the Board believe that a meaningful comparison between executive officer compensation and the compensation of other employees should be made, taking into account the relevant geographic location in which the executive officer is located, the executive officer’s role and scope of responsibility and the relevant geographic location of employees under the executive officer’s area of responsibility. Therefore, in addition to external benchmarking, the Compensation Committee and the Board reviewalso reviews relevant internal ratios between executive officer compensation and the compensation of other employees, includingspecifically the average and median values of other employee compensation, in Israel and other relevant geographies and its potential effect on ourthe Company’s labor relations.
Previousrelations in connection with the review and existingapproval of compensation arrangements: When considering the compensation package of anto executive officer, the Compensation Committee and the Board may consider the previous and existing compensation arrangements of such individual and his or her scope of responsibility.officers.
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 37
| 42 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Executive Compensation
In addition, see “Additional Compensation Information—2017 Pay Ratio” set forth below.IV. COMPONENTS OF OUR COMPENSATION PROGRAM
Risk Considerations
While the Board has overall responsibility for risk oversight, each of the standing committees of the Board regularly assesses risk in connection with executing its responsibilities. Therefore, the Compensation Committee assesses the potential risks arising from our compensation program, policies and practices. The Compensation Committee coordinates with our legal, human resources and other departments, considers shareholder feedback and interests and consults with its compensation consultant. The Compensation Committee reviewed and discussed the assessment for 2017. The Compensation Committee determined that our compensation program, policies and practices do not create risks that are reasonably likely to have a material adverse effect on Teva.
IV. Components of Our Compensation Program
20172020 Components in General
The HR and Compensation Committee, Board and shareholders selectedeach participated in the selection of the components of compensation set forth in the chart below to achieve our stated executive officer compensation program objectives. The majority of the compensation of each executive officer is variable and at-risk and a significant portion is subject to the achievement of performance goals in order to be earned. The HR and Compensation Committee and the Board review all components of the compensation of executive officers in order to verify that theeach executive officer’s total compensation is consistent with our compensation philosophy and objectives. The majority ofobjectives and within the compensation of each executive officer is variable and at risk and subject to the achievement of performance goals in order to be earned.parameters set by our shareholder-approved Compensation Policy.
Element | Description | |||
Base Salary |
Fixed cash compensation
Determined based on each executive officer’s role, individual skills, experience, performance, external market value and internal equity |
Base salaries are intended to provide stable compensation to executive officers, allow Teva to attract and retain | ||
Short-Term Incentives: Annual Cash |
Variable cash compensation based on the level of achievement
Cash incentives are capped at a maximum of 200% of base salary if achievement level is at least 120% of performance goal
|
Annual cash | ||
38 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 43 | |
Executive Compensation
Element | Description | |||
Long-Term Incentives: Annual Equity-Based Compensation | Variable equity-based compensation
Performance Share Units
Awards are capped at
Restricted Share Units
| Equity-based compensation is used to foster along-term link between executive officers’ interests and the interests of Teva and its shareholders, as well as to attract, motivate and retain executive officers for the In 2020, the CEO received 70% of the value granted in the form of PSUs and 30% in the form of RSUs; all other executive officers received 50% of the value granted in the form of PSUs and 50% in the form of RSUs | ||
2020 Target Pay Mix
The target pay mix supports the core principles of our executive officer compensation philosophy of compensating for performance and aligning executive officers’ interests with those of Teva and its shareholders, by emphasizing short- and long-term incentives.
The following charts outline the HR and Compensation Committee and the Board’s allocation of annual target total direct compensation payable to the CEO and to other NEOs. The HR and Compensation Committee and the Board allocated compensation among (i) base salary, (ii) short-term annual cash incentive opportunity and (iii) long-term annual equity.
A sizeable majority of target total direct compensation is variable, at-risk pay, consistent with our pay-for-performance philosophy. Specifically, in 2020, 86% of Mr. Schultz’s target total direct compensation was at-risk compensation, and 79%, on average, of the target total direct compensation of our other NEOs was at-risk compensation. We consider compensation to be “at risk” if it is subject to performance-based payment or vesting conditions or if its value depends on stock price appreciation.
| 44 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Executive Compensation
Target Pay Mix
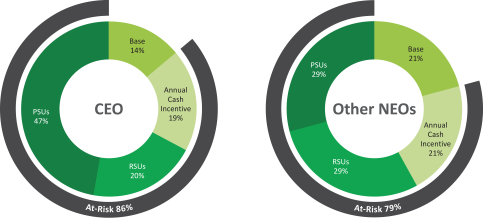
The percentages of target total direct compensation as calculated above are based on the annualized 2020 base salary, the 2020 annual cash incentive compensation opportunity (assuming achievement at the target level), and the grant date fair value of the annual equity grants. Each compensation element is outlined in more detail in the 2020 Summary Compensation Table and 2020 Grants of Plan-Based Awards table.
Base Salary
Purpose: Base salaries provide stable compensation to executive officers, allow Teva to attract and retain competentqualified global executive talent and maintain a stable leadershipmanagement team. Base salaries vary among executive officers, and are individually determined according to each executive officer’s areas of responsibility, role and experience based on a variety of considerations, including:which may include, inter alia, professional background (education, skills, expertise, professional experience and achievements and previous compensation arrangements, as relevant), external competitiveness, job criticality and internal fairness.
For 2020, there were no changes made to the annual base salaries for our CEO, CFO (disregarding any effects of changes in foreign exchange rates), and EVP, Global R&D compared to 2019. The HR and Compensation Committee adjusted the annual base salary of our EVP, North American Commercial, to bring it more in line with the market levels.
Executive | Annualized ($) | Annualized ($) | 2019-2020 % Change | ||||||||||||
Kåre Schultz | $ | 2,000,000 | $ | 2,000,000 | 0% | ||||||||||
Eli Kalif | $ | 657,494 | $ | 681,281 | 4% | (1) | |||||||||
Dr. Hafrun Fridriksdottir | $ | 720,000 | $ | 720,000 | 0% | ||||||||||
Brendan O’Grady | $ | 700,000 | $ | 800,000 | 14% | ||||||||||
Eric Drapé | N/A | $ | 708,010 | N/A | |||||||||||
| (1) | Mr. Kalif is paid in Israeli shekels. The difference in salary shown is due to fluctuations in exchange rates. |
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | ||
Adjustments to base salary: The Compensation Committee and the Board may periodically consider and approve base salary adjustments for executive officers. The main considerations for a salary adjustment are similar to those used in initially determining base salary, but may also include a change of role or responsibilities, recognition for professional achievements, regulatory or contractual requirements,
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 39
Executive Compensation
budgetary constraints or market trends. The Compensation Committee and the Board also consider the previous and existing compensation arrangements of the executive officer whose base salary is being considered for adjustment.
Annual Cash Incentives
Purpose:The annual cash incentive component aims to ensure that our executive officers are aligned in reachingincentivized to reach our short- and long-termannual goals. Annual cash incentives are designed to provide a significantpay-for-performance element of our executive compensation package.
Annual cash incentives:Payout eligibility and levels of individual annual cash incentives are determined based on actual financial and operational results, as well as individual performance. Following approval of the Company’s annual operating plan each calendar year, the Compensation Committee and the Board, following the CEO’s recommendations, determine the performance measures, taking into account ourshort- and long-term goals, as well as our compliance and risk management policies. The Compensation Committee and the Board may also determine any applicable super-measures that must be met for entitlement to the annual cash incentive (all or any portion thereof) and the formula for calculating any annual cash incentive payout, with respect to each calendar year, for each executive officer.
In special circumstances, as determined by the Compensation Committee and the Board (e.g., regulatory changes and significant changes in our business environment), the Compensation Committee and the Board may modify the objectives and/or their relative weights during the calendar year.
Parameters:Individual annual cash incentive parameters are determined by the Compensation Committee and the Board, taking into account our short- and long-term goals, as well as our risk management policy.
|
40 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Executive Compensation
The annual cash incentive parameters are intended to drive motivation and performance higher, while the maximum payout ceiling provides a risk management mechanism that assists in protecting Teva from excessive risk taking to achieve short-term results that could expose us to risk in the long-term, and aligns target setting with ourpre-defined risk profile.
In the event of an executive officer’s termination of service or employment where such executive officer served Teva for less than 12 months, he or she will not be entitled to an annual cash incentive, unless otherwise determined by the Compensation Committee and Board.
2017 Annual Cash Incentives
As provided in our Compensation Policy, annual cash incentives are designed to ensure that our executive officers are aligned in reaching our short- and long-term goals. Annual cash incentives are therefore a strictlypay-for-performance compensation element,package, as payout eligibility and levels are determined based on actual financial and operational company and business unit results,performance, as well as individual performance.
As provided inPursuant to our Compensation Policy, which serves as a shareholder approved framework, executive officer target annual cash incentive is capped at 100% of annual base salary, and as described above, ourfor the CEO, target annual cash incentive is capped at 150% of annual base salary. In addition, the maximum annual cash incentive payout cannot exceed 200% of target annual cash incentive.
The 2020 annual cash incentive plan for our executive officers takes the form of cash awards that are earned based onone-year performance. This structure aligns our executive officers’ interests with those of our shareholders by providingoffers incentives to the executive officers to achieveaccomplish certain short-term financial results that the HR and operational results established by theCompensation Committee and Board view as vital tokey steps in the execution of our overall business strategy.strategy, with the intent ultimately of increasing shareholder value.
For 2017,The amount of the Compensation Committee and the Board reviewed our company performance against our 2017 objectives in order to make determinations regarding whetherpayout, if any, payouts were due under our 2017 executive officers’ annual incentive plan. Due to the fact that our financial results were significantly below our original financial targets for the year, the Compensation Committee and the Board determined not to make any payouts under the executive officers’ annual cash incentive plan for 2017. Below we provide additional information about the design and operation of the 2017 annual cash incentive plan for executive officers.
Annual Cash Incentive Calculation Methodologyis determined as follows:
Eligible Salary | X | Target Incentive %
| X | Overall Performance Factor % |
|
=
Annual Cash Incentive Plan Payout |
Target Opportunities
The amount ofHR and Compensation Committee and the Board determine the target annual cash incentive for the executive officers, including the CEO and our other NEOs, is determined as follows. First, the Compensation Committee determines a target cash incentive opportunity available to each NEO by taking the individual’s base salary and multiplying it by the individual’s target incentive percentage.
Second, for each of the Company-, business unit- The HR and individual-level performance results, a weighted-average approach is used. As shown below, Company-level performance measures consist of financial measures and operational measures. Each component of the financial and operational measures has a weighting,Compensation Committee and the Compensation Committee determines the aggregate Company-level weighted average performance relative to target. Similarly, for business-unit level performance measures, each component is assigned a weighting, and the Compensation Committee determines the aggregate business-unit level weighted average performance relative toBoard did not change the target (exceptincentive percentages for the CEO, whose annual cash incentive is determined based on only Company- and individual-level measures). Finally, for individual-level measures, the Compensation Committee determines the individual performance rating based on achievement of individual goals.our NEOs from 2019 levels.
Executive | 2020 Target Annual Cash Incentive (% of Base Salary) | ||||
Kåre Schultz | 140 | % | |||
Eli Kalif | 100 | % | |||
Dr. Hafrun Fridriksdottir | 100 | % | |||
Brendan O’Grady | 100 | % | |||
Eric Drapé | 100 | % | |||
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 41
| 46 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Executive Compensation
Third, the Compensation Committee determines an overall performance factor. The Compensation Committee determines this overall performance factor by calculating the weighted average of the performance factors for Company-, business unit- and individual-level performance. There are slightly different potential factors for the CEO and the other executive officers, as described below. If the overall performance factor is below the overall threshold, then the performance factor will be zero (and the individual will not receive an annual cash incentive). If the overall performance factor is between the overall threshold and overall maximum, the individual’s overall performance factor will be determined by linear interpolation. If the overall performance factor is above the maximum, the maximum performance factor will be used.
Finally, the Compensation Committee takes the target cash incentive opportunities of the executive officers, including the CEO, and multiplies them by the applicable overall performance factor of the person to determine the actual cash incentive to be paid. The Compensation Committee then approves and presents the Company-, business unit- and individual-level achievement relative to target performance measures, the calculation of performance factors and the determination of incentive amounts to the Board for its review and approval.Performance Measures
The HR and Compensation Committee and the Board establishedused the followingsame performance measure categories, Company Financial and Individual, and weightings for 2017:
CEOas in 2019:
Category
| Weighting
|
| ||||||||
Company Financial | 75% | 50% Non-GAAP EPS |
| |||||||
25% Free Cash Flow |
| |||||||||
Individual | 25% | See Below | ||||||||
Other Executive Officers
|
|
| ||||
Company | ||||||
| ||||||
| ||||||
| ||||||
| ||||||
Company-Level: Financial Measures.: The HR and Compensation Committee and the Board believe that certain financial measures are key performance indicators of the present and future prospects of our business and key drivers of shareholder value, and selected the following financial measures for use in the annual cash incentive program:plan:
| ∎ |
| Non-GAAP |
42 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Executive Compensation
| ∎ | Free Cash |
These performance measures were selected because they focus management on metrics that align with our most critical strategic priorities of servicing debt, controlling expenses and improving profitability, and give a clear line of sight into how achieving operating goals drives Teva performance and generates rewards.
The HR and Compensation Committee and the Board used the non-GAAPNon-GAAP measures used for investor guidance as performance metrics in structuring our annual cash and our performance-based long-term equity incentive programs. The use of these measures is not intended to replace comparable GAAP measures as set forth in our consolidated financial statements. We believe that thesenon-GAAPNon-GAAP measures are helpful to management and investors as measures of operating performance because they exclude various items that do not relate to or are not indicative of operating performance. Please see “Item 7—Management’s Discussion
Target, Threshold and Analysis—SupplementalNon-GAAP Income Data” to our Annual Report on Form10-K for the year ended December 31, 2017 for reconciliations of these measures to the most directly comparable GAAP measuresMaximum Company Performance Levels. The HR and other required disclosures.
The table below shows Company-level financial performance measures and their weightings approved by the Compensation Committee and the Board set the 2020 threshold, target and maximum performance levels at levels that were considered rigorous, aggressive and challenging, attainable only with strong performance, and that took into account the relevant risks and opportunities.
The HR and Compensation Committee and the Board increased the target for Non-GAAP EPS by 6% over the 2017 annual cash incentive plan:2019 actual result and by 2% over the 2019 goal, and held the target for Free Cash Flow flat compared to 2019.
The HR and Compensation Committee and the Board did not make any adjustments to the targets during the year and did not exercise any discretion as a result of the COVID-19 pandemic.
|
| |||||
| ||||||
| ||||||
| ||||||
Company-Level: Operational Measures: Of
Executive Compensation
In developing our 2020 business plan and corresponding incentive plan performance metric targets, which were based on the 80% (CEO) or 60% (other executive officers)business plan and were aligned to the ranges of performance measures that are set atour outlook as communicated to investors in February 2020, the Company level, operational metrics constituted 30% of those respective amounts. TheHR and Compensation Committee and the Board believe that operational measures representmade key steps onassumptions, including:
| ∎ | Anticipated continued decline in COPAXONE revenue from $1.5 billion in 2019 to approximately $1.2 billion in 2020 due to an expected increase in generic competition; |
| ∎ | Anticipated continued increase of AUSTEDO revenue in the U.S. from $412 million in 2019 to $650 million in 2020; and |
| ∎ | Anticipated continued increase of global AJOVY revenue from $96 million in 2019 to $250 million in 2020. |
After setting the path to achieving shorttargets, the HR and long-term strategic objectives and value creation. For 2017, the Compensation Committee and the Board selected operationalalso set the threshold and maximum performance levels. To achieve threshold performance, 85% of target performance for each Company performance metric must be achieved, which is a rigorous and challenging level of achievement that must be met. An achievement percentage of less than 85% of target for either Non-GAAP EPS or Free Cash Flow would result in no annual cash incentive payment for executive officers. The HR and Compensation Committee and the Board set the maximum level of performance at 120% of target for each Company performance metric, a level that presented a significant challenge requiring exceptionally strong performance.
In spite of the impact of COVID-19 on the world economy and on our operations, we achieved 101% of our 2020 Non-GAAP EPS target and 106% of our 2020 Free Cash Flow target.
Weighting | Performance Metric | Threshold (85%) | Target (100%) | Maximum (120%) | Actual Results | % Achievement | |||||||||||||||||||||
50% | Non-GAAP EPS | $ | 2.17 | $ | 2.55 | $ | 3.06 | $ | 2.57 | 101 | % | ||||||||||||||||
25% | Free Cash Flow | $ | 1.7B | $ | 2.0B | $ | 2.4B | $ | 2.1B | 106 | % | ||||||||||||||||
| 48 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Executive Compensation
Individual Measures. The remaining 25% of the measures under the 2020 annual cash incentive plan were individual performance measures established by the HR and Compensation Committee and the Board early in the year. These measures included components specific to the nature of each executive officer’s position and area of responsibility. The HR and Compensation Committee and the Board evaluated performance with respect to the individual measures by determining an individual performance rating and individual performance achievement percentage, which was then used as follows:a component for determining the overall performance factor.
| Individual Goals |
Achievement | ||
Kåre Schultz | ∎ Achieving global sales targets overall and for new generic and specialty products (20% of 25% individual goal weighting)
∎ Achieving targets related to quality and Environmental, Health and Safety | 110% | ||
Eli Kalif |
∎ Ensuring compliance standards are met ∎ Achieving global gross margin targets ∎ Completing other critical function initiatives | 110% | ||
Dr. Hafrun Fridriksdottir | ∎ Ensuring compliance standards are met ∎ Achieving key generic and specialty products approval targets ∎ Achieving specialty product milestones targets ∎ Achieving generic products submissions targets | 105% | ||
Brendan O’Grady | ∎ Ensuring compliance standards are met ∎ Achieving regional gross margin targets ∎ Achieving regional sales targets ∎ Achieving targets related to employee engagement and retention | 115% | ||
Eric Drapé | ∎ Ensuring compliance standards are met ∎ Achieving global gross margin targets ∎ Achieving customer service and launch supply targets ∎ Achieving targets related to quality and Environmental, Health and Safety | 110% | ||
Overall Performance Factor and Payout Calculation
Overall Performance Factor.The HR and Compensation Committee and the Board calculated an overall performance factor for each executive officer by taking the weighted average of the achievement percentages of the Company financial measures and the individual measures and converting that weighted average to an overall performance factor based on the table below.
Weighted Average Level of Achievement of Objectives | Overall Performance Achievement % (1) (2) | Overall Payout Performance Factor: Potential Annual Cash Incentive | ||
Below Threshold |
Below 85% (below 90% for CEO) |
0% (no annual cash incentive payment) | ||
Threshold |
85% (90% for CEO) |
25% | ||
Target |
100% |
100% (140% for CEO) | ||
Maximum Cash Incentive |
120% |
200% |
|
| |||
|
| |||
| ||||
Teva Pharmaceutical Industries Ltd. | ||||
Business Unit Level: Of the 20% of performance measures that are set at the Business Unit level for executive officers (other than the CEO), the commercial business units have measures that include the following components:
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 43
Executive Compensation
The global function units, such as Finance, Human Resources and Legal, have operational measures that include components specific to their nature.
Individual Level: The remaining 20% of the measures under the annual cash incentive plan are individual performance measures established by the Compensation Committee and the Board early in the year in the following areas:
Strategy measures are primarily related to key planned strategic actions, such as transformation. Collaboration, culture and leadership measures are generally related to cross business unit collaboration, talent development and building organizational capability, and personal development. The Compensation Committee and the Board evaluate performance with respect to the individual measures using a rating system that equates to a level of performance, which is then used as a component for determination of the overall performance factor.
Overall Performance Factor:The Compensation Committee and the Board then determine the weighted average of the performance factors for Company-, business unit- and individual-level performance. Based on the weighted average performance, the overall performance factor is then determined based on the following table for each executive officer, and for the CEO, the former CEO and the former interim CEO:
|
|
| ||
|
|
| ||
|
|
| ||
|
|
| (1) | Payouts for performance for the CEO |
| (2) | Payouts for performance for other executive officers are determined linearly based on a straight-line interpolation of the applicable payout range (i.e., |
| (3) | Payout as a percentage of target for the |
44 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Executive Compensation
As described above, theThe HR and Compensation Committee and the Board reviewed our companyCompany financial and individual performance against our 2017 objectives. Due to the fact that our financial results were significantly below our original financial targets for the year, the Compensation Committee and the Board determined notobjectives in order to make determinations regarding whether any payouts were due under the executive officers’our annual cash incentive plan for 2017.
plan. The table below sets forth the calculation of the annual cash incentive planoverall performance achievement percentage and performance factor for our interim CEO, CEO and other NEOs whichNEOs:
Executive | Non-GAAP EPS % Achievement (50% weighting) | Free Cash Flow (25% weighting) | Individual % Achievement (25% weighting) | Overall Performance Achievement % | Overall Factor % | ||||||||||||||||||||
Kåre Schultz | 101% | 106% | 110% | 104% | 109% | ||||||||||||||||||||
Eli Kalif | 101% | 106% | 110% | 104% | 121% | ||||||||||||||||||||
Dr. Hafrun Fridriksdottir | 101% | 106% | 105% | 103% | 115% | ||||||||||||||||||||
Brendan O’Grady | 101% | 106% | 115% | 106% | 128% | ||||||||||||||||||||
Eric Drapé | 101% | 106% | 110% | 104% | 121% | ||||||||||||||||||||
Payout Calculation.The HR and Compensation Committee and the Board then took the target award opportunity and applied the overall payout performance factor to determine the total 2020 annual incentive plan payout for the CEO and each NEO. This amount is reflected in the“Non-Equity Incentive Plan Compensation” column of the Summary Compensation Table presented below in under the “Additional Compensation Information” section.
Name | Eligible Base ($) | Target Annual Cash Incentive (% of Base Salary) | Target Award ($) | Overall Performance Factor | Payout ($) | Cash Incentive Payout as a % of Base Salary | ||||||||||||||
Current NEOs
| ||||||||||||||||||||
Kåre Schultz
| $
| 2,000,000
|
|
| 140%
|
| $
| 2,800,000
|
| 0%
| $
| 0
|
| 0%
| ||||||
Michael McClellan (1)
| $
| 219,519
|
|
| 100%
|
| $
| 219,519
|
| 0%
| $
| 0
|
| 0%
| ||||||
Dr. Carlo de Notaristefani
| $
| 836,400
|
|
| 100%
|
| $
| 836,400
|
| 0%
| $
| 0
|
| 0%
| ||||||
Dr. Hafrun Fridriksdottir (1)
| $
| 630,577
|
|
| 100%
|
| $
| 630,577
|
| 0%
| $
| 0
|
| 0%
| ||||||
Mark Sabag
| $
| 604,637
|
|
| 100%
|
| $
| 604,637
|
| 0%
| $
| 0
|
| 0%
| ||||||
Former NEOs
| ||||||||||||||||||||
Erez Vigodman (2)
|
| N/A
|
|
| N/A
|
|
| N/A
|
| N/A
|
| N/A
|
| N/A
| ||||||
Dr. Yitzhak Peterburg (1)
| $
| 1,199,750
|
|
| 140%
|
| $
| 1,679,650
|
| 0%
| $
| 0
|
| 0%
| ||||||
Eyal Desheh (3)
| $
| 408,300
|
|
| 100%
|
| $
| 408,300
|
| 0%
| $
| 0
|
| 0%
| ||||||
Dr. Rob Koremans (4)
| $
| 783,215
|
|
| 100%
|
| $
| 783,215
|
| 0%
| $
| 0
|
| 0%
| ||||||
Dr. Michael Hayden (4)
| $
| 1,071,000
|
|
| 100%
|
| $
| 1,071,000
|
| 0%
| $
| 0
|
| 0%
| ||||||
Executive | Eligible Base ($) |
Target | Target Award ($) | Overall Payout Performance Factor(*) | Payout ($) | ||||||||||||||||||||
Kåre Schultz
|
$
|
2,000,000
|
|
|
140%
|
|
$
|
2,800,000
|
|
|
109%
|
|
$
|
3,055,920
|
| ||||||||||
Eli Kalif
|
$
|
681,281
|
|
|
100%
|
|
$
|
681,281
|
|
|
121%
|
|
$
|
826,599
|
| ||||||||||
Dr. Hafrun Fridriksdottir
|
$
|
720,000
|
|
|
100%
|
|
$
|
720,000
|
|
|
115%
|
|
$
|
828,576
|
| ||||||||||
Brendan O’Grady
|
$
|
776,923
|
|
|
100%
|
|
$
|
776,923
|
|
|
128%
|
|
$
|
991,198
|
| ||||||||||
Eric Drapé
|
$
|
708,010
|
|
|
100%
|
|
$
|
708,010
|
|
|
121%
|
|
$
|
859,028
|
| ||||||||||
| (*) | Percentages have been rounded to the nearest whole percentage. |
| 50 | ||
Executive Compensation
Long-Term Incentive Equity-Based Compensation
Purpose: The third and largest main component of the executive compensation program is long-term equity incentives.
Equity-based compensation is intended to incentivize and reward for future long-term performance, as reflected by the market price of our shares and/or other performance criteria, and is used to foster a long-term link between executive officers’ interests and the interests of Teva and its shareholders, as well asshareholders. Equity-based compensation is also intended to attract, motivate and retain executive officers for the long-term by:long term by (i) providing them with a meaningful interest in Teva’s share performance; (ii) linking equity-based compensation to potential and sustained performance; and (iii) spreading benefits over a longer performance cycle through the vesting period mechanism.
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 45
ExecutivePursuant to our Compensation
Equity-based awards: Equity-based awards are generally granted to executive officers on an annual basis, and at other times Policy, which serves as a shareholder approved framework, the Compensation Committee and the Board deem appropriate, including for newly hired or promoted executive officers or due to special retention needs. Notwithstanding the foregoing, the Compensation Committee and the Board may determine with respect to a specific year that no equity-based awards will be granted to all or any particular executive officers.
Parameters: Equity-based awards are granted pursuant to Teva’s 2015 Long-Term Equity-Based Incentive Plan, and/or any other long-term incentive plan(s) that we may adopt in the future, and generally on terms and conditions provided for therein and as determined by the Compensation Committee and the Board, provided that any such terms and conditions are consistent with the following:
The monetary grant value of executive officers’ equity-based awards is determined bythree years from the Compensation Committeedate of grant (partial vesting can occur before), the maximum monetary grant date fair value of annual equity-based awards granted to the CEO cannot exceed $11 million at target and to any other executive officer $4.5 million at target, and the Board, taking into account, among other things, our pay mix targets,maximum number of shares settled for a performance-based equity award shall not exceed 250% of the desired mixtarget number of equity-based vehicles, the executive officer’s contribution to Company performance, desired competitive compensation levelsshares granted.
Equity Vehicles and dilution or pool limits. When establishing the monetary grant value, the Compensation Committee and the Board also determine the mix of equity-based vehicles for each grant, which may include various types of time-based and performance-based equity-based vehicles, such as share options, restricted share units, performance share units and/or other share-based awards. The value of each type of equity-based vehicle is determined in accordance with accepted valuation and accounting principles, as they apply to the relevant type of equity-based vehicle, and might differ from the value determined for other purposes.Mix
The mix of equity-based vehiclesHR and the relative weight assigned to each type of equity-based vehicle out of the total equity-based grant is structured to enhance the executive officers’ commitment to increasing Company and shareholder value and is designed to encourage balanced and effective business risk-taking. The Compensation Committee and the Board may change the distribution and elements of the equity mix from time to time.
46 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Executive Compensation
Caps on equity-based compensation:
2017 Long-Term Equity Incentives—Annual Grant
As described above, the Compensation Committee and the Board intend for long-term equity-based compensation to reward executive officers based on our future performance, as reflected by the market price of Teva’s shares, to foster a long-term link between executive officers’ interests and the interests of Teva and its shareholders, as well as to attract, motivate and retain executive officers for the long-term by:
In making determinations about 2017 long-term equity incentive grants to executive officers, the Compensation Committee and the Board considered, among other things:
The sizes of the grants to executive officers vary based on the factors above. The portion of executive officer compensation that is composed of these equity vehicles is “at risk” and directly aligned with shareholder value creation.
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 47
Executive Compensation
For the 2017 long-term equity incentive grants to executive officers, the Compensation Committee and the Board used the termsequity vehicles and mix set forth in the following table:
Type of Long-
| Proportion
| Vesting
| Performance
| Rationale for Use of Performance Metric
| ||||
1) 2020-2022 Net Revenue (50%) | 1) Primary measure of top line growth | |||||||
Performance Share Units | CEO; 50% for other executive officers | 2) 2020-2022 |
| |||||
| ||||||||
| 3) | 3) Strong performance, as measured by the other two operating metrics, is fully rewarded only if it also results in above
| |||||||
Restricted Share Units | CEO; 50% for other executive officers |
| ||||||
|
| N/A | N/A |
The PSU performance measures were selected because (i) Free Cash Flow andNon-GAAP EPS focus management on metrics that align with our most critical strategic priorities of servicing debt, controlling expenses and improving profitability, (ii) relative TSR is an important measure for shareholders and (iii) they give recipients a clear line of sight into how executing on operating measures drives the achievement of performance and earning awards.
The Compensation Committee and the Board utilize RSUs to encourage ownership and retention while immediately aligning executive officers’ interests with those of our shareholders, and options are meant to focus executive officers on share price appreciation.
PSU Calculation Methodology
In connection with the 2017 PSU grants, the number of shares earned by the CEO, former interim CEO, and to the other executive officers will be determined in two steps as follows.
48 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 51 | |
Executive Compensation
ThereThe HR and Compensation Committee and the Board have structured the mix of equity vehicles and the relative weight assigned to each type to motivate performance against long-term targets, stock price appreciation over the long term and to encourage ownership and retention while aligning executive officers’ interests with those of our shareholders. The RSUs are twocomplementary to the PSUs because they have upside potential but deliver some value even during periods of market or stock price underperformance, providing a retention incentive and reinforcing an ownership culture and commitment to Teva.
Performance Metrics
Financial Measures
Change to Financial Measures in 2020. Consistent with our overall shift from being in a mode of turnaround and stabilization to positioning for growth, for 2020, the HR and Compensation Committee and the Board reviewed and re-evaluated the performance measures under the long-term incentive equity program, and changed the Net Debt Reduction metric to Net Revenue. This was done because, having made meaningful progress in step 1, 2017-2019Non-GAAP EPSreducing the Company’s leverage, the HR and 2017-2019 Free Cash Flow, eachCompensation Committee and the Board sought to focus executive officers more on top line growth.
The 2020-2022 PSU performance measures were selected because:
| ∎ | Net Revenue, as discussed above, focuses management on growing our top line, particularly as we look to pivot to more of a growth phase; |
| ∎ | Non-GAAP Operating Profit aligns with our most critical strategic priorities of controlling expenses and improving profitability; and |
| ∎ | Relative TSR is an important measure because TSR ties executive officer compensation with shareholder value creation, aligns the interests and experience of executive officers with those of Teva and its shareholders and filters out macroeconomic and other factors that are not within management’s control. |
All metrics give recipients a clear line of sight into how achieving operating goals drives Teva performance and generates rewards.
Before the conclusion of the three-year performance period, we do not publicly disclose our specific performance measure targets and the corresponding minimums and maximums because of the potential for competitive harm from such disclosure. These measures are competitively sensitive and would reveal information about our view of our anticipated trajectory, which is weighted an equal 50%not otherwise public. The HR and Compensation Committee and the Board believe that they have set performance goals at rigorous and challenging levels so as to require significant effort and achievement by our executive officers to be attained, and that such goals have been established in light of our internal forecast as well as the macroeconomic and industry environments. After the end of the performance period, the targets and achievement relative to such targets will be disclosed.
Target, Threshold and Maximum Performance Levels. Similar to the annual cash incentive plan, for the PSUs, the HR and Compensation Committee and the Board defined payout levels representing the number of PSUs to be earned by executive officers based on the level of actual performance relative to the three-year targets.
| 52 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Executive Compensation
For each of thesethe two measures, Net Revenue and non-GAAP Operating Profit, the HR and Compensation Committee determines the Company’s performance for the measureachievement percentage for the three-year period. The Company’s performance with respect to each measureachievement percentage is compared to the target for the measure, and the proportion of achievement isthen converted to a factoran earning percentage as set forth below.
Level of Achievement of Objectives(*) | % Achievement of Target | Earning Percentage | Performance Achievement % | Earning Percentage | ||||||||||||||||
Below Threshold | Below 85% | 0% | ||||||||||||||||||
Threshold
| Up to 85%
| 0%
| 85% | 25% | ||||||||||||||||
Target
| 100%
| 100%
| 100% | 100% | ||||||||||||||||
Maximum
| 120%
| 200%
| 120% | 200% | ||||||||||||||||
(*) Linear interpolation will be used to determine the applicable earning percentage.
| (*) | Linear interpolation will be used to determine the applicable earning percentage between levels. |
The HR and Compensation Committee then calculates the average of the earning percentages for the two performance measures.
In step two, thisRelative TSR Modifier.The HR and Compensation Committee and the Board introduced a TSR modifier in the PSU design beginning in 2017. They continue to view the inclusion of Total Shareholder Return as critical because it ties executive officer compensation to the shareholder experience and the creation of shareholder value, and it aligns the interests of executive officers with those of Teva and its shareholders. By measuring our stock performance relative to peers, it mitigates the impact of macroeconomic factors, both positive and negative, that affect the industry and/or stock price performance and are beyond the control of management, and it provides rewards that are more directly aligned with performance through different economic cycles.
The average of the earning percentages is multiplied by a modifier that has been determined based on our relative TSR performance for the three yearthree-year period as set forth below. The Company’s TSR is ranked relative to our Peer Group; the HR and Compensation Committee and the Board believe that the Peer Group is an appropriate comparator group that is broadly representative in terms of its size, industry, geographic footprint and employee base. See “—III. Compensation Determination Process—Compensation Peer Group and Peer Selection Process” for a list of the peer group companies used for this purpose.
Level of Achievement of Relative TSR(*) | Relative TSR Ranking | Modifier | |||||
Threshold | Up to 25th percentile |
average of the earning percentages) | |||||
Target | 50th percentile | 100% | |||||
Maximum
| 75thth percentile or above
| 120%
| |||||
| (*) | Linear interpolation will be used to determine the applicable modifier. |
The product of (1) the average of the earning percentages and (2) the relative TSR modifier is multiplied by the target number of PSUs granted to the CEO, former interim CEO, and to each of the executive officers respectively, to determine the final number of sharesPSUs earned by each individual, except thatindividual. For example, if the numberCompany’s TSR rank is less than or equal to the 25th percentile, then the average of sharesthe earning percentages is multiplied by 80% (equivalent to be issued may not exceed 200%a reduction of 20%) to determine the final performance factor and then multiplied by the target number of PSUs to determine the final number of earned PSUs. If the Company’s TSR rank is at the 75th percentile or above, then the average of the earning percentages is multiplied by 120% (equivalent to an increase of 20%) and then incorporated into the calculation.
TheUltimately, the HR and Compensation Committee approves and presents the performance achievement relative to target performance measures,percentages, the calculation of the average earning percentage and the TSR modifier, and the determination of the number of PSUs earned PSUsby each executive officer to the Board for its review and approval.
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 49
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 53 | |
Executive Compensation
2017 Long-Term Incentive Award Values—Annual Grant2020 Grants of PSUs and RSUs
In connection withmaking determinations ofabout 2020 long-term equity incentive grants to executive officers, the appropriate level of annual equity grants for 2017, theHR and Compensation Committee and the Board took into accountconsidered, among other things: sustained performance; criticality of contributions to Teva; comparison against our Peer Group; the executive officer’s role, skills, experience and development; internal fairness among executive officers; current value of prior granted equity and pay mix. The sizes of the grants to executive officers vary based on these factors outlined above as well as information regarding the absolute levels and allocations at the companies in the Peer Group. The Compensation Committeeportion of executive officer compensation that is composed of these equity vehicles is “at risk” and the Board determined that it was consistentdirectly aligned with our performance-based compensation philosophy and appropriate to structure the equity grants to executive officers such that (1) 33% are earned and vest only if the CEO and executive officers achieve specified levels of performance as measured by certain metrics, and (2) 67% are earned and vest over four years. shareholder value creation.
The following table sets forth the 20172020 annual awardgrant date fair values at target approved by the HR and Compensation Committee, the Board and shareholders for the CEO and by the HR and Compensation Committee and the Board for the other NEOs.
Name | PSUs ($) (1/3) | RSUs ($) (1/3) | Share Options ($) (1/3) | Total ($) | ||||||||||||||||
Current NEOs
|
| |||||||||||||||||||
Kåre Schultz
| $
| 1,999,998
|
| $
| 1,999,993
|
| $
| 2,000,009
|
| $
| 6,000,000
|
| ||||||||
Michael McClellan (1)
|
| N/A
|
| $
| 132,331
|
| $
| 132,329
|
| $
| 264,660
|
| ||||||||
Dr. Carlo de Notaristefani
| $
| 866,657
|
| $
| 866,659
|
| $
| 866,688
|
| $
| 2,600,004
|
| ||||||||
Dr. Hafrun Fridriksdottir
| $
| 499,979
|
| $
| 499,979
|
| $
| 500,047
|
| $
| 1,500,005
|
| ||||||||
Mark Sabag
| $
| 533,310
|
| $
| 533,319
|
| $
| 533,375
|
| $
| 1,600,004
|
| ||||||||
Former NEOs
|
| |||||||||||||||||||
Erez Vigodman (2)
|
| N/A
|
|
| N/A
|
|
| N/A
|
|
| N/A
|
| ||||||||
Dr. Yitzhak Peterburg
| $
| 1,499,992
|
| $
| 1,499,999
|
| $
| 1,500,009
|
| $
| 4,500,000
|
| ||||||||
Eyal Desheh
| $
| 666,666
|
| $
| 666,649
|
| $
| 666,686
|
| $
| 2,000,001
|
| ||||||||
Dr. Rob Koremans
| $
| 833,326
|
| $
| 833,319
|
| $
| 833,361
|
| $
| 2,500,006
|
| ||||||||
Dr. Michael Hayden | $
| 666,666
|
| $
| 666,649
|
| $
| 666,686
|
| $
| 2,000,001
|
| ||||||||
Executive | PSUs ($) (1) | RSUs ($) (1) | Total ($) (1) | |||||||||
Kåre Schultz (2) | $ | 7,000,000 | $ | 3,000,000 | $ | 10,000,000 | ||||||
Eli Kalif (3) | $ | 850,000 | $ | 850,000 | $ | 1,700,000 | ||||||
Dr. Hafrun Fridriksdottir | $ | 1,000,000 | $ | 1,000,000 | $ | 2,000,000 | ||||||
Brendan O’Grady | $ | 1,150,000 | $ | 1,150,000 | $ | 2,300,000 | ||||||
Eric Drapé
| $
| 900,000
|
| $
| 900,000
|
| $
| 1,800,000
|
| |||
| (1) | Equity values have been rounded to |
| (2) | Please see “—Shareholder-Approved Amendment to CEO Terms of Office and Employment” below. |
| (3) | Amounts shown exclude the one-time sign-on RSU replacement grants. |
Consistent with historical practice, the dollar value allocated to PSUs was converted to a number of units, based on the grant date fair market value on the grant dates as determined in accordance with the Financial Accounting Standards Board Accounting Standards Codification Topic 718. The dollar amount allocated to RSUs was converted to a number of shares using the fair market value on the grant date. The dollar amount allocated to share options was converted to a number of shares using the Black Scholes valuation method as of the grant dates.
2015-20172018-2020 Performance Share Unit Payout
In 2015,2018, the HR and Compensation Committee and the Board granted PSUs with performance-based vesting requirements for the three-year performance period 2015-2017. The threshold level of achievement was 90%, the target level of achievement was 100%, and the maximum level of achievement was 120% of the PSU performance goals as defined below, with a maximum payout of 150% of the target number of PSUs. Payouts for performance between threshold and target and between target and maximum were determined based on a straight-line linear interpolation of the applicable payout range (i.e., 10% for each percentile change in performance between threshold and target and 2.5% for each percentile change in performance between target and maximum).
The Compensation Committee and the Board set the three-year performance targets (“PSU Performance Goals”) for net revenues andnon-GAAP operating profit at the beginning of 2015, subject to adjustment for the effect of changes in currency exchange rates. The Compensation Committee and the Board set
50 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Executive Compensation
these targets based on certain assumptions about our performance. Pursuant to the 2015 award agreements, the Compensation Committee and the Board have the discretion to adjust (increase or decrease) the PSU Performance Goals and their relative weights if one or more of the following items of gain, loss, profit or expense, having a material impact on the PSU Performance Goals, is: (i) determined to be extraordinary, unusual ornon-recurring in nature; (ii) related to changes in accounting principles under U.S. GAAP or tax laws; (iii) related to currency fluctuations; (iv) related to productivity initiatives or new business initiatives; (v) related to discontinued operations that do not qualify as a segment of business under U.S. GAAP; or (vi) attributable to the business operations or assets of any entity acquired or licensed by the Company during the fiscal year, to the extent the Compensation Committee or the Board, as applicable, determines that the adjustment is necessary or advisable to preserve the intended incentives and benefits of the PSUs, or if such adjustments were reflected in our publicnon-GAAP financial results.
2018-2020. In connection with evaluating our achievementthe 2018 PSU grants, the number of PSUs earned by the NEOs who were executive officers at the time of the 2015-2017grants has been determined in two steps as follows.
In step one, there were two Company financial performance metrics,measures, 2018-2020 Non-GAAP EPS and 2018-2020 Free Cash Flow, each of which was weighted 50%. For each of these two measures, the HR and Compensation Committee and the Board determined thatthe Company’s performance achievement percentage for each year in orderthe three-year period relative to the target set according to the annual business plan for each year. The HR and Compensation Committee and the Board then calculated the average annual performance achievement percentage for the PSU Performance Goalsthree-year period. The average three-year performance achievement percentage was then converted to operate as they were intended to, they would make adjustments by increasingan earning percentage based on the targets due primarily to the 2016 acquisition of Actavis Generics. The aggregate effect of these adjustments was an increase of approximately 16% in the net revenue target and an increase of approximately 22% in thenon-GAAP operating profit target. For the performance period, our actual net revenue achievement was 91% and our actualnon-GAAP operating profit achievement was 86%, resulting in a weighted average achievement of 88.5%. This level of achievement was below the threshold level of performance of 90%, resulting in no payout, a 122% decrease compared to what would have been if the upward adjustments had not been taken into account.following:
Weighting | Performance Metric | Target ($MM) Excluding FX | Adjusted Target (100%) ($MM) | Actual Results ($MM) | % Achievement | |||||||||||||
50% | Net Revenue | 58,654 | 67,992 | 62,045 | 91 | % | ||||||||||||
50% | Non-GAAP Operating Profit | 17,557 | 21,461 | 18,406 | 86 | % | ||||||||||||
Weighted Average: |
| 88.5 | % | |||||||||||||||
Based on this outcome, the NEOs did not earn any Teva shares in respect of their 2015-2017 PSU awards:
Level of Achievement of Objectives(*) | % Achievement of Target | Earning Percentage | ||||||||
Below Threshold | Below 85% | 0% | ||||||||
Threshold | 85% | 25% | ||||||||
Target | 100% | 100% | ||||||||
Maximum
| 120%
| 200%
| ||||||||
Name | Target Award (# of PSUs) | Payout Factor | Final Award (# of PSUs) | |||||||||
Current NEOs |
| |||||||||||
Kåre Schultz (1) | N/A | N/A | N/A | |||||||||
Michael McClellan (1) | N/A | N/A | N/A | |||||||||
Dr. Carlo de Notaristefani | 16,838 | 0 | % | 0 | ||||||||
Dr. Hafrun Fridriksdottir (1) | N/A | N/A | N/A | |||||||||
Mark Sabag | 12,628 | 0 | % | 0 | ||||||||
Former NEOs |
| |||||||||||
Erez Vigodman (2) | 30,869 | 0 | % | 0 | ||||||||
Dr. Yitzhak Peterburg (1) | N/A | N/A | N/A | |||||||||
Eyal Desheh (2) | 16,838 | 0 | % | 0 | ||||||||
Dr. Rob Koremans (2) | 17,773 | 0 | % | 0 | ||||||||
Dr. Michael Hayden (2) | 17,773 | 0 | % | 0 | ||||||||
Linear interpolation was |
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 51
Executive Compensation
| 54 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Performance, Promotion and OtherOne-Time Grants
In connection with a performance-based cash award earned by Dr. Fridriksdottir when she was an employee of Actavis Generics prior to its acquisition by Teva in 2016, we assumed the obligation to pay a cash incentive of $535,000 based on Actavis Generics shareholder return performance metrics. Also, pursuant to a legacy 2014 Actavis Generics retention plan that we also assumed, we fulfilled the assumed obligation under the plan by making payment of a $900,000 cash award to Dr. Fridriksdottir.
In connection with the promotion of Mr. McClellan to the position of Interim CFO in July 2017 (before his promotion to Executive Vice President, CFO in November 2017), we awarded Mr. McClellan aone-time promotion cash award of $202,500 in recognition of his increased responsibility.One-half of the award was paid in November 2017, and the remaining half was paid in February 2018. In order to secure the services of Mr. McClellan during a time of transition while he served as Interim CFO, we granted him 12,341 options, 4,091 RSUs, and a cash award totaling $67,500.One-half of the cash award will be paid in September 2018 and the remaining half will be paid in September 2019. The options and RSUs will vest in September 2019. All payments and vesting are subject to continued employment through the applicable vesting dates. These grants were part of a broader program to secure the services of key employees during a period of uncertainty for our Company.
In addition, in May 2017, we granted Dr. de Notaristefani 30,875 RSUs due to his significance and key role during the transition period and the importance of securing his services. The RSUs will vest in May 2019.
Information regarding thesign-on equity and cash grants for Kåre Schultz is provided in the “Leadership Transitions” section below.
Leadership Transitions
Appointment of Mr. Kåre Schultz as President and CEO
In September 2017, our Board successfully completed its global search process (with the assistance of a search firm) for our next President and CEO when it appointed Kåre Schultz to the position. In its search, the Board sought a leader with extensive global pharmaceutical experience and a strong track record in corporate turnarounds, as well as in driving growth and leading international expansion. Mr. Schultz is a seasoned leader in the health care industry with an extensive background leading global companies’ financial and restructuring initiatives.
Since 2015, and prior to his appointment as our President and CEO, Mr. Schultz served as the President and CEO of H. Lundbeck A/S, which he joined as the company was facing the loss of critical patents. Mr. Schultz conducted a top to bottom evaluation of the business and implemented a robust turnaround strategy that involved cutting operating costs while targeting new product launches. As a result of his leadership, H. Lundbeck A/S is on track to achieveall-time high revenue and earnings with significant stock price appreciation and increased market cap. Prior to joining Lundbeck, Mr. Schultz worked for nearly three decades at Novo Nordisk, where he served in a number of leadership roles, including Chief Operating Officer, Vice President in Product Supply and Director of Product Planning and Customer Services in the Diabetes Care Division.
Based on this outstanding profile, our Board selected Mr. Schultz as the best candidate to lead Teva presently and to participate in the establishment of, and steer the execution of, our strategic and operational goals. The Board appointed Mr. Schultz as President and CEO effective November 1, 2017, and he joined the Board at that time.
52 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Executive Compensation
The termsHR and Compensation Committee and the Board then calculated the average of the employment agreement with Mr. Schultz were negotiated in order to induce him to acceptearning percentages for the Board’s offer to become our President and CEO attwo performance measures.
In step two, this critical time, including relocation to our headquarters in Israel. The Board was mindfulaverage of the challenges currently facing our Company in its various business segments, product lines and markets, the advent of generic competition for one of our key branded specialty products, the fierce competition for talented executives in the pharmaceutical industry and the extreme pressure on, and vast duties of, the leader of an international organization of the size and complexity of Teva in approving the components of the compensation package, including the annual base salary, target annual incentive, annual equity grants, and inducement equity grants and cash awards to Mr. Schultz. The Board also took into account the difficulty not just in identifying an individual with the desired skills and experience, but also retaining the person throughout the period of transition and significant change being drivenearning percentages was multiplied by the Board. Accordingly, the Board, with the assistance of its independent compensation consultant, developed a compensation packagemodifier that considered pay structures for CEOs at peer companies, and which includes pay that depends on long-term Company performance as well as the opportunity to accumulate a significant ownership interest in Teva upon the creation of sustained shareholder value.
In light of all of these factors, we entered into an employment agreement with Mr. Schultz which provides for an initial employment term of five years, subject to automatic renewal for subsequentone-year periods (or until the second anniversary following a change in control of the Company, if later than the otherwise applicable term end date) until a notice ofnon-renewal is provided or other termination circumstances occur.
Under the employment agreement, Mr. Schultz received an annual base salary of $2 million, a performance-based target annual incentive opportunity equal to 140% of his annual base salary (and a maximum opportunity of 200% of his annual base salary), annual long-term equity incentives with a total target grant date fair value of $6 million with vesting terms similar to other senior executive officers, a meaningful portion of which are performance-based, and the same employee benefits as are provided to similarly situated senior executives of the Company.
Upon commencing employment on November 1, 2017, Mr. Schultz received the followingsign-on equity awards over the five year term of the agreement, which are designed to align his interests with those of Teva and its shareholders over the long term (like our stock ownership guidelines to which he is subject): (i) a restricted share unit award with a grant date fair value of $5 million (aswas determined based on the closing price of Teva’s shares on the date priorour TSR performance relative to the announcement of Mr. Schultz’s hire), which will vest and settle in equal installments on the third, fourth and fifth anniversaries of the employment commencement date (the “Effective Date”); and (ii) twosign-on performance share unit awards, each with a target grant date fair value of $7.5 million (as determined based on the closing price of Teva’s shares on the date prior to the announcement of Mr. Schultz’s hire), which will be earned based on the achievement of performance goals related to the absolute increasecompanies included in the price of Teva’s shares over three- and five-year periods following the Effective Date, which range frompeer group used as a 16% increase to a 158% increasereference point for 2018 compensation decisions for the three-year performance period, and from a 28% increase to a 385% increase foras set forth below.
Level of Achievement of Relative TSR(*) | Relative TSR Ranking | Modifier | ||
Threshold | Up to 25th percentile | 80% (i.e., 20% less than unmodified average of the earning percentages) | ||
Target | 50th percentile | 100% | ||
Maximum | 100th percentile | 150% | ||
| (*) | Linear interpolation was used to determine the applicable modifier. |
The product of (1) the five-year period, and generally vest on the third, fourth and fifth anniversariesaverage of the Effective Date (inearning percentages and (2) the caserelative TSR modifier was multiplied by the target number of PSUs granted to the award with a three-year performance period) and onNEOs, to determine the fifth anniversaryfinal number of the Effective Date (in the case of the award with a five-year performance period). In addition, Mr. Schultz received asign-on cash award of $20 million, which will vest and be paid in two equal installments three and six months following the Effective Date. In connection with his relocation to Israel, Mr. Schultz will also receive a housing reimbursement and certain relocation benefits.
As the grant date value of equity awards for accounting purposes depends on, among other things, stock price, the actual grant date fair values of thesesign-on equity awardsPSUs earned by each individual, except that appear in our Summary Compensation Table are lower than the intended target values described in the preceding paragraph since the number of units was set based on the closing price of Teva’s shares on the date priorPSUs to the announcement of Mr. Schultz’s hire, but the grant date fair valuebe issued could not exceed 300% of the awards for accounting purposestarget number of PSUs.
The HR and Compensation Committee and the Board approved an average earning percentage of 158% which was then subject to a relative TSR modifier, which adjusted the earning percentage downward by 20%, resulting in a modified earning percentage of 126%.
Weighting | Performance Metric | Year | Target | Actual Results | % Achievement | Earning % | |||||||||||||||||||||
|
|
|
| 2018 | $ | 2.50 | $ | 2.92 | 117 | % |
| ||||||||||||||||
50% | Non-GAAP EPS | 2019 | $ | 2.40 | $ | 2.40 | 100 | % |
| ||||||||||||||||||
|
|
|
| 2020 | $ | 2.55 | $ | 2.57 | 101 | % |
| ||||||||||||||||
|
| ||||||||||||||||||||||||||
|
|
|
| Average |
|
|
|
|
|
| 106 | % | 131% | ||||||||||||||
|
|
|
| 2018 | $ | 2.8B | $ | 3.7B | 132 | % |
| ||||||||||||||||
50% | Free Cash Flow | 2019 | $ | 1.8B | $ | 2.1B | 113 | % |
| ||||||||||||||||||
|
|
|
| 2020 | $ | 2.0B | $ | 2.1B | 106 | % |
| ||||||||||||||||
|
| ||||||||||||||||||||||||||
|
|
|
| Average |
|
|
|
|
|
| 117 | % | 185% | ||||||||||||||
Weighted Average: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 158% | |||||||||||
Relative TSR Modifier (-20%, reflecting <25th percentile) |
|
|
|
|
|
|
| 80% | |||||||||||||||||||
Final Earning % |
|
|
|
|
|
|
| 126% | |||||||||||||||||||
Based on this outcome, the NEOs earned Teva PSUs in respect of their 2018-2020 PSU awards as follows:
Executive | Target Award (# of PSUs) | Final Earning Percentage | Final (# of PSUs) | ||||||||||||
Kåre Schultz | 179,104 | 126% | 226,173 | ||||||||||||
Eli Kalif (1) | N/A | N/A | N/A | ||||||||||||
Dr. Hafrun Fridriksdottir | 83,731 | 126% | 105,736 | ||||||||||||
Brendan O’Grady | 53,731 | 126% | 67,852 | ||||||||||||
Eric Drapé
|
| 51,890
|
|
| 126%
|
|
| 65,527
|
| ||||||
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 53
| (1) | Mr. Kalif was hired subsequent to the 2018 grant date. |
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 55 | |
Executive Compensation
were determined when they were actually granted.Shareholder-Approved Amendment to CEO Terms of Office and Employment
As required by the Israeli Companies Law, at our 2020 annual meeting of shareholders, our shareholders approved an amendment to the Terms of Office and Employment of our CEO, including the amount of an equity grant.
During his then-tenure to date, Mr. Schultz had successfully executed our two-year restructuring plan, reduced the Company’s spend base by $3 billion, reduced debt and overseen the launch of new products. Through these achievements, Mr. Schultz demonstrated his commitment to taking actions aimed at generating shareholder value and positioned the Company for a return to growth. The HR and Compensation Committee believed that fixingand the number of units was appropriate and consistent with the aforementioned focus on aligning executives’ compensation with long-term shareholder value creation. See “—Additional Compensation Information—2017 Grants of Plan-Based Awards.”
Appointment of Mr. Michael McClellan as CFO; Previous Appointment as Interim CFO
Effective as of November 27, 2017, we entered into an employment agreement with Mr. McClellan. For the preceding two years, Mr. McClellan served as the Senior Vice President and CFO of Teva’s Global Specialty Medicines division. Prior to joining Teva, he was the U.S. CFO at Sanofi. The agreement providesBoard expressed their view that Mr. McClellan will be employed as Executive Vice President, CFO, until his death, disability, termination with or without cause or resignation with or without good reason. He will continue his international assignment in Amsterdam, Netherlands,Schultz was best-suited to lead the organization forward at that critical juncture.
The shareholders approved the amendment that included the following changes:
| ∎ | Extension of Employment Agreement Term. In seeking to ensure the services of Mr. Schultz through a longer time horizon, shareholders approved extending the initial term of Mr. Schultz’s employment by one year to a sixth year, from November 1, 2022 to November 1, 2023. |
| ∎ | Align Target Annual Total Direct Compensation with Market Median. In general, the HR and Compensation Committee and the Board seek to align executive officer compensation with the market median range in order to be able to attract, motivate and retain experienced executive talent who are critical to our long-term success, and especially so with the CEO. |
The HR and on or about September 1, 2018, he will relocate to our corporate headquarters in Israel. The agreement provides for an initial base salary of $700,000. Mr. McClellan is eligible to be considered for anCompensation Committee and the Board reviewed the target annual cash incentive with a target of 100% of his then current base salary, and for equity-based awards under our equitytotal direct compensation plan.
In July 2017, in connection with the promotion of Mr. McClellan to the position of Interim CFO (before his promotion to Executive Vice President, CFO in November 2017), we awarded Mr. McClellan aone-time promotion cash award of $202,500 in recognition of his increased responsibility.One-half of the CEO and each of its components and determined that the CEO’s target annual total direct compensation and target long-term incentive equity award was paid in November 2017, and the remaining half was paid in February 2018.prior to 2020 were positioned below market median. In order to retainbring target annual total direct compensation to the services of Mr. McClellan during a time of transition while he served as Interim CFO, we granted him 12,341 options, 4,091 RSUs, and a cash award totaling $67,500.One-halfmarket median range for the duration of the cashCEO’s tenure, and in accordance with our pay for performance and shareholder alignment philosophy, shareholders approved increasing the target grant date fair value of the CEO’s annual long-term incentive equity award will be paid in September 2018 and the remaining half will be paid in September 2019. The options and RSUs willby $4 million (from $6 million to $10 million annually), all of which would vest in September 2019. All payments and vesting aresolely subject to continued employment throughachievement of pre-established performance metrics designed to further align the applicable vesting dates.
AppointmentCEO’s interests with those of Dr. Hafrun Fridriksdottir as Executive Vice President, Global R&D
On November 27, 2017, we appointed Dr. Hafrun Fridriksdottir as Executive Vice President, Global R&D. Since February 2017, she served as Executive Vice President, President of Global Generics R&D, after serving as Senior Vice President and President of Global Generics R&D since August 2016. Prior to joining Teva, Dr. Fridriksdottir served as Senior Vice President of Global Generics R&D of Allergan plc, where she held several positions of increasing responsibilityTeva’s shareholders. The resulting total target long-term incentive equity grant remains below the limit set forth in the Actavis group within Allergan.
On June 18, 2017, we entered into an employment agreement with Dr. Fridriksdottir.Company’s Compensation Policy as approved by shareholders. The agreement provides that Dr. Fridriksdottir will serve in a senior R&D position until her death, disability, termination with or without cause or resignation with or without good reason. The agreement provided for an initial base salary of $720,000. Dr. Fridriksdottir is eligible to be considered for antarget annual cashlong-term incentive (prorated for 2017 and under the applicable plan prior to being appointed an executive officerequity award for the time period before such appointment),CEO is now 70% performance-based PSUs and for equity-based awards under our equity compensation plan.
Separation of former President and CEO Erez Vigodman
In February 2017, Erez Vigodman stepped down as President and CEO.
Pursuant to the terms of our employment agreement with Mr. Vigodman, in connection with his termination of employment, Mr. Vigodman was entitled to receive nine months’ notice, payments associated with termination as required pursuant to Israeli law, certain previously accrued obligations, and a payment that, together with severance amounts accumulated in his existing pension insurance funds, equals the product of twice his monthly base salary multiplied by the number of his years of service. Mr. Vigodman is also receiving an amount equal to eighteen times his monthly base salary in consideration for compliance with certainnon-competition covenants.30% time-based RSUs.
| ∎ | Post-Termination Equity Treatment. As part of the extension of the employment agreement, shareholders also approved a revision to Mr. Schultz’s employment agreement to change the treatment of outstanding long-term incentive awards upon certain qualifying terminations of employment. Following the effective date of the employment agreement amendment, in the event Mr. Schultz incurs a termination of employment with the Company (a) by the Company without “cause”, (b) by Mr. Schultz for “good reason”, (c) following the Company’s decision not to renew the employment agreement, or (d) by Mr. Schultz following his decision not to renew the employment agreement due to his retirement, defined as a cessation to work as an employee in a full-time managerial capacity for any for-profit organization, any then-outstanding long-term incentive equity grants (both time and performance-based equity grants) would continue to vest following such a termination of employment in accordance with their terms. Continued vesting following a qualifying termination of employment will be subject to Mr. Schultz’s continued compliance with the non-compete, non-solicitation, non-disparagement and confidentiality covenants contained within his employment agreement. In addition, following the effective date of the employment agreement amendment, the non-compete covenant also applies following a termination of employment due to the Company’s decision not to renew the employment agreement and following Mr. Schultz’s decision not to renew the employment agreement due to his retirement. |
54 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
| 56 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Executive Compensation
Under his employment agreement, Mr. Vigodman is also entitledNew Hire and Legacy One-Time Grants
The HR and Compensation Committee and the Board do not generally intend to continued vesting of equity-based awards for twelve months following terminationprovide one-time grants except in a very judicious and an extension oflimited manner in rare circumstances as warranted by the exercisesituation. The HR and Compensation Committee and the Board view any such grants to the NEOs as a special and exceptional nonrecurring event to meet the Company’s needs during a specific period of outstanding optionsor for a period of ninety days after the twelve month period.
Appointmentspecific purpose. The HR and Separation of Dr. Yitzhak Peterburg as Interim PresidentCompensation Committee and CEO
Dr. Yitzhak Peterburg served as Interim President and CEO from February 2017 until November 2017. Prior to that, Dr. Peterburg served as Chairman of the Board since January 2015. When Mr. Schultz began his service as Presidentcontinue to prudently and CEO on November 1, 2017, Dr. Peterburg continuedcarefully evaluate our compensation program to serve as a memberensure that it aligns the interests of our Boardexecutive officers and then resigned fromshareholders and links their pay to the Company’s performance.
In 2020, the HR and Compensation Committee and the Board on December 12, 2017.
Pursuantdid not approve any new one-time grants to Dr. Peterburg’s terms of employment, during his service as Interim Presidentany NEOs. As approved in 2019 and CEO, Dr. Peterburg was compensated inpreviously disclosed, Mr. Kalif received a manner comparable to our former President and CEO, Mr. Vigodman, subject to certain differences relating to his interim status, as described below.
Dr. Peterburg’s compensation included (i) a monthly base salary of 488,250 Israeli shekels (approximately $135,608 using a 2017 average monthly exchange rate of 3.60 shekels per U.S. dollar); (ii) an annual cash incentive(pro-ratedsign-on for service for the partial period of the year) with an annual target amount equal to 140% of annual base salary and a maximum amount equal to 200% of annual base salary; (iii) an annual equity award with an aggregate target fair market value of $4.5 million under terms consistent with those of the previous President and CEO, with one third of the annual award being granted in the form of options to purchase Teva shares, one third in the form of PSUs and one thirdFebruary 2020 in the form of RSUs calculatedwith a grant date fair value of $250,000 in accordance with accepted valuationconsideration of certain equity grants from Mr. Kalif’s prior employer that were forfeited upon his resignation. These RSUs vest in three equal installments on the second, third, and accounting principles, as they applyfourth anniversaries of the grant date, subject to his continued employment through the relevant type of equity-based vehicle andapplicable vesting dates.
As previously disclosed, in accordance with Teva practice; and (iv) termination arrangements as described below.
PursuantDecember 2016, we made a cash retention award to Dr. Peterburg’s termsFridriksdottir of employment, in connection with his termination of employment, Dr. Peterburg is entitled to receive nine months’ notice, payments associated with termination as required pursuant to Israeli law, certain previously accrued obligations, and a payment that, together with severance amounts accumulated in his existing pension insurance funds, equals the product of twice his monthly base salary multiplied by the number of his years of service as Interim President and CEO.
Under his employment terms, Dr. Peterburg will also be entitled to continued vesting in full of all equity-based awards granted as Interim President and CEO and continued exercisability of vested options through their expiration dates.
Separation of former CFO Eyal Desheh
In July 2017, Eyal Desheh stepped down as Group Executive Vice President and CFO.
Pursuant to the terms of our employment agreement with Mr. Desheh, in connection with his termination of employment, Mr. Desheh is entitled to receive nine months’ notice, payments associated with termination as required pursuant to Israeli law, certain previously accrued obligations, amake-up payment equal to his monthly base salary multiplied by the number of his years of service, that together with severance amounts accumulated in his pension insurance fund account cannot exceed twice his monthly base salary multiplied by the number of his years of service, and eligibility to apro-rata annual cash incentive for the term active in position. Mr. Desheh is also receiving an amount equal to twelve times his monthly base salary, conditioned upon his undertaking not to compete with Teva for$750,000. We paid one year following termination.
Mr. Desheh is also entitled to continued vesting in full of equity-based awards and continued exercisability of vested options through their expiration dates due to our qualifying retirement and qualifying termination policy.
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 55
Executive Compensation
Separation of Dr. Rob Koremans
As a resultquarter of the new organizationcash award in January 2019 and leadership structure, on November 27, 2017,paid the remaining three quarters in January 2020. This award was granted to Dr. Rob Koremans stepped downFridriksdottir prior to her appointment as President and CEO, Global Specialty Medicines.
Pursuant to the terms of our employment agreement with Dr. Koremans, in connection with his termination of employment, Dr. Koremans is entitled to receive six months’ notice, a severance payment equal to 12 monthly salaries and target annual cash incentive (for a total of 24 monthly salaries).
Dr. Koremans is also entitled to continued vesting of equity-based awards until March 1, 2020 and continued exercisability of vested options through their expiration dates.
Separation of Dr. Michael Hayden
As a result of the new organization and leadership structure, on November 27, 2017, Dr. Michael Hayden stepped down as President of Global R&D and Chief Scientific Officer.
Pursuant to the terms of our employment agreement with Dr. Hayden, in connection with his termination of employment, Dr. Hayden is entitled to receive nine months’ notice, payments associated with termination as required pursuant to Israeli law, certain previously accrued obligations, a payment equal to 12 monthly salaries, a payment that, together with severance amounts accumulated in his existing pension insurance funds, equals the product of twice his monthly base salary multiplied by the number of his years of service, a payment equal to the premium for continued health insurance coverage for eighteen months following the termination date and certain relocation benefits in the event of a move back to Canada within one year following the termination date.
Dr. Hayden is also entitled to continued vesting of certain equity-based awards and continued exercisability of vested options through their expiration dates due to our qualifying retirement and qualifying termination policy.an executive officer.
SupplementalNon-GAAP Income Data
We utilize certainnon-GAAPNon-GAAP financial measures to evaluate performance, in conjunction with other performance metrics. The following are examples of how we utilize thenon-GAAPNon-GAAP measures:
| ∎ | our executives and Board use |
| ∎ | our annual budgets are prepared on a |
| ∎ | senior |
Non-GAAP financial measures have no standardized meaning and accordingly have limitations in their usefulness to investors. We provide thisnon-GAAP data because our executives believe that the data provide useful information to investors. However, investors are cautioned that, unlike financial measures prepared in accordance with U.S. GAAP,non-GAAP measures may not be comparable with the calculation of similar measures for other companies. Please see “Item 7—Management’s Discussion
For the definitions of Non-GAAP financial measures and Analysis—Supplementala reconciliation of these items to the most directly comparable financial measures calculated and presented in accordance with GAAP, reference is made to the section captioned “Supplemental Non-GAAP Income Data” toin the Annual Report onCompany’s Form10-K for the year ended December 31, 2017 for2020.
Other Elements of Compensation
We generally provide to our executive officers the same benefits that are provided to all employees, including certain health and welfare benefits and other benefits. In addition, our executive officers are provided with certain additional information.benefits, intended to be competitive with the practices of companies in our Peer Group.
56 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 57 | |
Executive Compensation
Health and Welfare Benefits
We offer health and welfare benefits to all eligible employees, including all executive officers, which are tailored to each location’s competitive market. Health and welfare benefits may include medical, dental, prescription drug, vision, life insurance, accidental death and dismemberment, short- and long-term disability coverage and an employee assistance program.
Retirement and Other Local Benefits
Israel
Israeli law generally requires severance pay equal to one month’s salary for each year of employment upon the termination of an employee’s employment due to retirement, death, termination without cause and other circumstances as defined under Israeli law. We make monthly contributions on behalf of our executive officers on Israel payroll to pension plans. These funds provide a combination of pension allowance and/or insurance and severance pay benefits to the executive officers. We contribute 7.5% of an NEO’s monthly salary to the pension component (including disability insurance) and 8.33% of the NEO’s monthly salary to the severance component, and the employee contributes an amount between 6% and 7% of the monthly salary to the pension component. The contributions to the severance component are on account of Teva’s obligation to pay severance upon termination as referenced above. Our CEO and EVP, Global Operations are entitled to similar contributions on behalf of the Company as a pension contribution and on account of severance. Accordingly, a substantial part of our statutory severance obligation is covered by these monthly contributions.
Generally, in addition, our NEOs on Israel payroll (excluding those on relocation), like all of our employees in the country, are entitled to participate in a study fund plan, pursuant to which each employee who participates in the plan contributes an amount equal to 2.5% of his or her monthly salary to the study fund and Teva contributes to this fund or pays 7.5% of his or her monthly salary.
North America
Our North American subsidiaries mainly provide various defined contribution plans for the benefit of their employees. Under these plans, contributions are based on specified percentages of pay. In addition, Teva USA offers a supplemental deferred compensation plan to eligible employees. The plan is a nonqualified plan which is intended to work as a complement to the qualified 401(k) Retirement Savings Plan. The plan has been designed to address the “retirement gap” that many senior level employees face, primarily due to IRS-imposed limits on qualified plans and IRAs.
Expatriate Benefits / International Assignment and Relocation Benefits
Teva provides benefits to our employees who either accept an expatriate assignment or relocate internationally. The benefits are designed to provide ongoing assignment management and physical relocation support services. These benefits can vary depending on the nature of the assignment or relocation, but generally include a housing allowance, transportation support, a cost of living allowance (where applicable), home leave, global health insurance, and Company-paid education for approved dependents in locations where public education is not suitable. Additionally, we provide tax preparation and tax support services, dependent on the nature of the assignment (e.g., tax equalization for home-based assignments or tax gross up of relocation benefits and ongoing assignment allowances for host-based assignments), as well as immigration services to manage compliance within all global jurisdictions. The purpose of these benefits is to keep our expatriate employees “economically neutral” for the costs associated with living and working outside their home country, with the goal that they are not financially advantaged or disadvantaged as a result of relocating to another country and incurring associated taxes.
| 58 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Executive Compensation
Details regarding benefits and perquisites specific to each NEO can be found in the footnotes to the 2020 Summary Compensation Table set forth below under “Additional Compensation Information.”
V. Additional Compensation Policies and PracticesADDITIONAL COMPENSATION POLICIES AND PRACTICES
EquityStock Ownership PolicyGuidelines
Teva and its shareholders are best served by executives and directors that manage the business with a long-term perspective. Therefore, we adopted sharestock ownership guidelines, as we believe sharestock ownership is an important tool to strengthen the alignment of interests among shareholdersour executive officers and directors and our shareholders, to reinforce executive officers. officers’ commitment to Teva and to demonstrate Teva’s commitment to sound corporate governance.
The policy provides that Teva expects the applicable required level of equitystock ownership to be satisfied by our executive officers within five years, and by our directors within six years, of the later of the date the guidelines were adopted in June 2016 or the date of appointment as an executive officer.officer or director. If an executive officer’s holding requirement increases because of a change in annual base salary, or if a director’s holding requirement increases because of a change in annual cash retainer, the executive officer is expected to achieve the higher holding requirement within one year, and the director within two years, of the date of the increase.
The HR and Compensation Committee receives periodic reports of the ownership achieved by each executive officer.officer and director. For purposes of determining compliance with the guidelines, the value of an executive officer’sofficer or director’s share holdings is based on the closing price of Teva’s American Depositary Shares reported on the principal U.S. national securities exchange on which the shares are listed (currently the NYSE) on the last trading day of the year.
The following table represents the required salary multiples:ownership guidelines are as follows:
Current Position | ||||||
| ||||||
CEO | 6x | |||||
All other executive officers | 3x | |||||
Directors | 5x annual cash fee(*) | |||||
| (*) |
|
The value of all of the following types of Teva shares or sharestock options owned by, or granted to, an executive officer or director qualifies toward the participant’s attainment of the target multiple of pay:
| ∎ | shares owned outright by the executive officer or director or jointly with, or separately by, his or her immediate family members residing in the same household; |
| ∎ | shares held in a grantor trust or under a similar arrangement for the economic benefit of the executive officer or director or his or her immediate family members residing in the same household; |
| ∎ | shares held in any Teva retirement plan; |
| ∎ | unvested time-based |
| ∎ | the |
At the last measurement date, all NEOs are in compliance with our stock ownership guidelines, or fall within the period to attain the guideline level of stock ownership.
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 59 | |
Executive Compensation
Clawback Policy
Our executive officers are required to return any compensation paid to them on the basis of results included in financial statements that turned out to be erroneous and that were subsequently restated, during the three yearthree-year period following filing thereof. In such case,cases, compensation amounts will be returned net of taxes that were withheld thereon, unless the executive officer has reclaimed or is able to reclaim such tax payments from the relevant tax authorities (in which case the executive officer will also be obligated to return such tax amounts). We will publicly disclose the general circumstances of any repayment or forfeiture of compensation from our executive officers under our clawback policy, and the aggregate amounts repaid or forfeited, if required by applicable law or regulation, or if we have previously disclosed the underlying event giving rise to the repayment or forfeiture, unless such disclosure would, as determined by our HR and Compensation Committee or Board, raise legal or privacy concerns with respect to those individuals involved or would not be in the best interests of our shareholders.
In addition, in the event that it is discovered that an executive officer engaged in conduct that resulted in a material inaccuracy in Teva’s financial statements or caused severe financial or reputational damage to Teva, or in the event that it is discovered that an executive officer breached confidentiality and/ornon-compete obligations to Teva (as determined by the Compensation Committee)Company), the Compensation Committee shall haveCompany has broad remedial and disciplinary authority. Such disciplinary action
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 57
Executive Compensation
or remedy would vary depending on the facts and circumstances, and may include, without limitation, (i) termination ofterminating employment, (ii) initiating an action for breach of fiduciary duty, and (iii) seeking reimbursement of performance-based or incentive compensation paid or awarded to the executive officer.officer, including by means of an offset to, or cancellation of, outstanding grants or opportunities. The Compensation CommitteeCompany will determine applicable terms to enforce repayment of clawback amounts and may modify this clawback policy in accordance with applicable law and regulations.
Anti-Hedging and PledgingAnti-Pledging Policies
DirectorsOur directors and executive officers are prohibited from hedging their equity-based awards and any other Teva securities held by them (whether they are subject to transfer restrictions or not), such as purchasing or selling options on Teva securities, purchasing or selling puts, calls, straddles, equity swaps or other derivative securities linked to Teva’s securities, or engaging in “short” sales on Teva securities. This policy applies to each director and each executive officer until one year after the director’s or executive officer’s termination or retirement.
Our employees are also strongly discouraged from participating in any transaction in which profit is earned through a decline in value of Teva securities, such as short sales or hedges. Directors and executive officers are subject to certain restrictions onprohibited from pledging or using their equity-based awards and any other Teva securities held by them (whether they are subject to transfer restrictions or not) as collateral for loans.
Risk Considerations and Assessment
Our compensation elements are designed to include mechanisms that reduce incentives to expose Teva to imprudent risks that may harm the Company or our shareholders in the short- and long-term. This is achieved by using tools such as (i) placing maximum limits on short- and long-term incentives; (ii) measuring performance using key performance indicators that are designed to reduce incentives to take excessive risks; (iii) using compensation vehicles with diverse performance measures; (iv) granting a mix of equity-based compensation types that have long-term vesting schedules, which tie the awards to a longer performance cycle; (v) requiring clawback of compensation payments in certain circumstances; and (vi) requiring compliance with meaningful stock ownership guidelines.
While the Board and the Audit Committee have overall responsibility for risk oversight, each of the standing committees of the Board regularly assesses risk in its area of oversight in connection with executing its responsibilities. Thus, the HR and Compensation Committee assesses the potential risks arising from our compensation program, policies and practices. The HR and Compensation Committee
| 60 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Executive Compensation
coordinates with our legal, human resources and other departments, considers shareholder feedback and interests and consults with its compensation consultant. The HR and Compensation Committee reviewed and discussed the assessment for 2020. The HR and Compensation Committee determined that our compensation program, policies and practices do not create risks that are reasonably likely to have a material adverse effect on Teva.
Compensation Policy Requirements
Pursuant to the Israeli Companies Law, arrangements between Teva and its Office Holders must generally be consistent with the Compensation Policy. However, under certain circumstances, we may approve an arrangement that is not consistent with the Compensation Policy, if the arrangement is approved by a majority of our shareholders who participate and vote, provided that (i) the majority includes a majority of the votes cast by shareholders who participate and vote (abstentions are disregarded) who (A) are not controlling shareholders and (B) do not have a personal interest in the matter, or (ii) the votes cast against the arrangement by shareholders who are not controlling shareholders and who do not have a personal interest in the matter who participate and vote constitute two percent or less of the voting power of the Company (a “special majority”).
In addition, pursuant to the Israeli Companies Law, the Terms of Office and Employment of Office Holders generally require the approval of the HR and Compensation Committee and the Board. The Terms of Office and Employment as applicable to directors, including with respect to other positions in the Company, further require the approval of the shareholders by a simple majority. The Terms of Office and Employment with respect to a CEO (who is not a director) generally require the approval of the shareholders by the special majority referenced in the immediately preceding paragraph.
Under certain circumstances, if the Terms of Office and Employment of Office Holders who are not directors are not approved by the shareholders, where such approval is required, the HR and Compensation Committee and the Board may nonetheless approve such terms. In addition, non-material amendments of the Terms of Office and Employment of Office Holders who are not directors may be approved by the HR and Compensation Committee only and non-material amendments of the Terms of Office and Employment of Office Holders who are not directors and excluding the CEO may be approved by the CEO only, provided such approvals are permitted under the Compensation Policy and consistent therewith.
Accordingly, pursuant to our Compensation Policy, the HR and Compensation Committee can authorize our CEO to approve changes in terms for any other executive officer with respect to any calendar year, provided that it does not exceed the value of such executive officer’s one-month base salary.
Tax Deductibility
Prior to the Tax Cuts and Jobs Act (the “TCJA”) signed into law in December 2017, Section 162(m) of the Internal Revenue Code of 1986, as amended (the “Code”), generally limited the corporate tax deduction for compensation paid to the CEO and the three other most highly compensated executives (other than the CFO) to $1.0 million annually, unless certain requirements were satisfied. To maximize the corporate tax deduction, incentive plans were designed so that certain awards under those plans would constitute “qualified performance-based compensation” for purposes of Section 162(m) of the Code and preserve our corporate tax deductibility for those amounts.
The TCJA contained significant changes to Section 162(m) of the Code, including the elimination of the performance-based compensation exception to Section 162(m) for corporate tax years beginning after December 31, 2017, and an expansion of employees covered by the provision. Section 162(m) now covers the CFO or any individual who served as the CFO in the relevant taxable year. In addition, once an individual becomes a covered employee under Section 162(m) for any taxable year beginning after
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 61 | |
Executive Compensation
December 31, 2016, this status carries forward to all future years, even in the event of the employee’s termination or death. The actTCJA provides limited transition relief for certain grandfathered “performance-based” compensation, specifying that compensation payable pursuant to a written binding contract which was in effect on November 2, 2017 and which was not modified in any material respect on or after that date will remain eligible for the “performance-based” paycompensation exception to Section 162(m) (i.e., may remain deductible even if in excess of $1 million). The U.S. Internal Revenue Service is expected to providehas provided some guidance on the application of the transition relief to specific situations. However, given the changes to Section 162(m), we expect that the U.S.-based tax deductibility of performance-based compensation in excess of $1.0 million will be less of a consideration for us when designing and implementing our executive officers’ compensation program in future years.
Other Benefits and Perquisites
We generally provideWhile the TCJA may limit the deductibility of compensation paid to our CEOcovered employees, the HR and executive officersCompensation Committee and the same benefitsBoard will—consistent with past practice—retain flexibility to design compensation programs that are provided to all employees, including certain retirement benefits, health and welfare benefits, and other benefits. In addition, our executive officers are provided with certain additional benefits, intended to be competitive with the practices of our peer companies.
58 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Executive Compensation
Our Compensation Policy provides that:
Benefit plans and perquisites have three main objectives:
Benefit plans and perquisites are intended to supplement cash compensation and often involvenon-monetary rewards, coverage of certainbusiness-related expenses, insurance, pension and savings plans and other deferred monetary savings. These benefits and perquisites may vary depending on geographic location and other circumstances. Global, regional and local units may develop their own benefit plans and procedures, consistent with Teva’s principles and guidelines and subject to any required Company approvals. Benefits and perquisites may include, in addition to benefits that are mandated by applicable law and/or generally provided to other employees (including related costs and expenses): car, transportation and accommodations, telecommunication devices, media and computer equipment and expenses, travel and relocation (includingfamily-related expenses, such as tuition and commuting) and life and medical insurance and benefits (including executive officers’ families).
Health and Welfare Benefits
We offer health and welfare benefits to all eligible employees, including the President and CEO and executive officers, which are tailored to each location’s competitive market. Health and welfare benefits may include medical, dental, prescription drug, vision, life insurance, accidental death and dismemberment, short- and long-term disability coverage and an employee assistance program.
Retirement and Other Local Benefits
Israel
Israeli law generally requires severance pay equal to one month’s salary for each year of employment upon the termination of an employee’s employment due to retirement, death, termination without cause (and other circumstances as defined under Israeli law). We make monthly contributions on behalf of our Israel-based executive officers to a pension plan known as Managers’ Insurance or to a Pension Fund. These funds provide a combination of pension allowance and/or insurance and severance pay benefits to the executive officers. We contribute 7.5% of the monthly salary to the pension component (including disability insurance) and 8.33% of the monthly salary to the severance component and the employee contributes an amount between 6% and 7% of salary to the pension component. These contributions are on account of Teva’s obligation to pay severance upon termination as referenced above. Our President and CEO is entitled to similar contributions on behalf of the Company as pension contribution and on account of severance. Accordingly, a substantial part of our statutory severance obligation is covered by these monthly contributions.
Generally, in addition, our Israel-based executive officers (excluding the current President and CEO), are entitled to participate in an study fund plan, pursuant to which each employee who participates in the plan contributes an amount equal to 2.5%best long-term interests of his or her monthly salary to the study fundTeva and Teva contributes 7.5%our shareholders, with deductibility of his or her monthly salary to this fund.
North America
Our North American subsidiaries mainly provide various defined contribution plans for the benefitcompensation being one of their employees. Under these plans, contributions are based on specified percentages of pay. In addition, Teva USA offers a supplemental deferred compensation plan to eligible employees. The plan is a nonqualified
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 59
Executive Compensation
plan which is intended to work as a complement to the qualified 401(k) Retirement Savings Plan. The plan has been designed to address the “retirement gap” that many highly compensated individuals face, primarily due to IRS imposed limits on qualified Plans and IRAs. Finally, certain executive officers located in the United States participate in a defined contribution supplemental executive retirement plan. No new executive officers are enrolled in this plan.
Expatriate Benefits / International Assignment and Relocation Benefits
Teva provides benefits to our employees, who either accept an expatriate assignment or relocate internationally. The benefits are designed to provide ongoing assignment management, where applicable, and physical relocation support services. These benefits can vary depending on the nature of the assignment or relocation, but generally include a housing allowance, transportation support, a cost of living allowance (where applicable), home leave, global health insurance, and company paid education for approved dependents in locations where public education is not suitable. Additionally, we provide tax preparation and tax support services, dependent on the nature of the assignment (e.g., tax equalization for home-based assignments or tax gross up of relocation benefits and ongoing assignment allowances for host-based assignments), as well as immigration services to manage compliance within all global jurisdictions.
Details regarding benefits and perquisites specific to each NEO can be found in the footnotes to the Summary Compensation Table set forth below under “Additional Compensation Information.”factors considered.
HR and Compensation Committee Report
The HR and Compensation Committee has reviewed and discussed this “Compensation Discussion and Analysis” section of this Proxy Statement with our executives. Based upon this review and discussions, the HR and Compensation Committee recommended to the Board that the “Compensation Discussion and Analysis” be included in this Proxy Statement.
This HR and Compensation Committee Report shall not be deemed to be incorporated by reference into any filing made by the Company under the Securities Act of 1933, as amended (the “Securities Act”) or the U.S. Securities Exchange Act of 1934, as amended, notwithstanding any general statement contained in any such filing incorporating this proxy statement by reference, except to the extent the Company incorporates such Report by specific reference.
Members of the HR and Compensation Committee:
Rosemary A. Crane, Chair
Gerald M. Lieberman
Jean-Michel Halfon
Nechemia (Chemi) J. Peres
Janet S. Vergis
60 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
| 62 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Executive Compensation
ADDITIONAL COMPENSATION INFORMATION
20172020 Summary Compensation Table
Name and Principal
| Year
| Salary
| Bonus
| Stock
| Option
| Non-Equity
| Change in
| All Other
| Total ($)
| ||||||||||||||||||||||||||||||||||||
Kåre Schultz President and Chief Executive Officer
|
|
2017
|
|
|
333,333
|
|
|
0
|
|
|
14,229,808
|
|
|
2,000,009
|
|
|
0
|
|
0
|
|
464,591
|
|
|
17,027,741
|
| ||||||||||||||||||||
Michael McClellan Executive Vice President, Chief Financial Officer
|
|
2017
|
|
|
397,058
|
|
|
101,250
|
|
|
199,260
|
|
|
195,515
|
|
|
0
|
|
0
|
|
199,579
|
|
|
1,092,662
|
| ||||||||||||||||||||
Dr. Carlo de Notaristefani Executive Vice President, Global Operations
|
|
2017 |
|
|
836,400 |
|
|
0 |
|
|
2,569,720 |
|
|
866,688 |
|
|
0 |
|
0 |
|
189,551 |
|
|
4,462,359 |
| ||||||||||||||||||||
| 2016 | 835,832 | 0 | 1,074,937 | 1,075,070 | 872,532 | 0 | 191,766 | 4,050,137 | |||||||||||||||||||||||||||||||||||||
|
| 2015
|
|
| 877,231
|
|
| 0
|
|
| 899,991
|
|
| 900,016
|
|
| 1,189,398
|
| 0
|
| 178,988
|
|
| 4,045,624
|
| |||||||||||||||||||||
Dr. Hafrun Fridriksdottir Executive Vice President, Global Research and Development
|
|
2017
|
|
|
696,346
|
|
|
900,000
|
|
|
999,957
|
|
|
500,047
|
|
|
535,000
|
|
0
|
|
77,492
|
|
|
3,708,842
|
| ||||||||||||||||||||
Mark Sabag Executive Vice President, Global Human Resources
|
|
2017
|
|
|
604,637
|
|
|
0
|
|
|
1,066,630
|
|
|
533,375
|
|
|
0
|
|
0
|
|
317,108
|
|
|
2,521,750
|
| ||||||||||||||||||||
Erez Vigodman Former President and CEO |
|
2017 |
|
|
1,378,702 |
|
|
0 |
|
|
0 |
|
|
0 |
|
|
0 |
|
0 |
|
1,455,704 |
|
|
2,834,406 |
| ||||||||||||||||||||
| 2016 | 1,528,437 | 0 | 2,249,948 | 2,250,061 | 0 | 0 | 478,671 | 6,507,117 | |||||||||||||||||||||||||||||||||||||
|
| 2015
|
|
| 1,363,692
|
|
| 0
|
|
| 1,649,948
|
|
| 1,650,060
|
|
| 2,253,581
|
| 0
|
| 435,942
|
|
| 7,353,223
|
| |||||||||||||||||||||
Dr. Yitzhak Peterburg Former Interim President and CEO
|
|
2017
|
|
|
1,534,467
|
|
|
0
|
|
|
2,999,991
|
|
|
1,500,009
|
|
|
0
|
|
0
|
|
737,071
|
|
|
6,771,538
|
| ||||||||||||||||||||
Eyal Desheh Former Group ExecutiveVice President and ChiefFinancial Officer
|
|
2017 |
|
|
831,428 |
|
|
0 |
|
|
1,333,315 |
|
|
666,686 |
|
|
0 |
|
0 |
|
1,369,101 |
|
|
4,200,530 |
| ||||||||||||||||||||
| 2016 | 779,399 | 0 | 1,124,934 | 1,125,072 | 591,775 | 0 | 269,231 | 3,890,411 | |||||||||||||||||||||||||||||||||||||
|
| 2015
|
|
| 733,863
|
|
| 0
|
|
| 899,991
|
|
| 900,016
|
|
| 1,110,824
|
| 0
|
| 253,068
|
|
| 3,897,762
|
| |||||||||||||||||||||
Dr. Rob Koremans Former President and CEO, Global Specialty Medicines
|
|
2017
|
|
|
783,215
|
|
|
0
|
|
|
1,666,644
|
|
|
833,361
|
|
|
0
|
|
0
|
|
1,807,975
|
|
|
5,091,195
|
| ||||||||||||||||||||
Dr. Michael Hayden Former President of Global R&D and Chief Scientific Officer
|
|
2017 |
|
|
1,071,000 |
|
|
0 |
|
|
1,333,315 |
|
|
666,686 |
|
|
0 |
|
0 |
|
1,152,537 |
|
|
4,223,538 |
| ||||||||||||||||||||
| 2016 | 1,071,000 | 500,000 | 1,149,956 | 1,150,055 | 970,202 | 0 | 2,638,012 | 7,479,225 | |||||||||||||||||||||||||||||||||||||
|
| 2015
|
|
| 1,050,000
|
|
| 500,000
|
|
| 949,967
|
|
| 950,034
|
|
| 1,608,239
|
| 0
|
| 1,045,992
|
|
| 6,104,232
|
| |||||||||||||||||||||
Name and Principal Position | Year | Salary ($) (1) | Bonus ($) (2) | Stock Awards ($) (3) | Option Awards ($) (4) | Non-Equity Incentive Plan Compensation ($) (5) | Change in Pension Value and Nonqualified Deferred Compensation Earnings ($) | All Other Compensation ($) (6) | Total ($) | ||||||||||||||||||||||||||||||||||||
Kåre Schultz President and Chief Executive Officer | 2020 | 2,000,000 | 0 | 9,999,970 | 0 | 3,055,920 | 0 | 668,628 | 15,724,518 | ||||||||||||||||||||||||||||||||||||
| 2019 | 2,000,000 | 0 | 5,999,977 | 0 | 2,869,720 | 0 | 726,867 | 11,596,564 | |||||||||||||||||||||||||||||||||||||
| 2018 | 2,000,000 | 20,000,000 | 4,499,977 | 1,500,019 | 3,790,360 | 0 | 679,519 | 32,469,875 | |||||||||||||||||||||||||||||||||||||
Eli Kalif Executive Vice President, Chief Financial Officer
| 2020 | 681,281 | 0 | 1,949,974 | 0 | 826,599 | 0 | 265,961 | 3,723,815 | ||||||||||||||||||||||||||||||||||||
| 2019 | 18,130 | 0 | 0 | 0 | 0 | 0 | 2,425 | 20,555 | |||||||||||||||||||||||||||||||||||||
Dr. Hafrun Fridriksdottir Executive Vice President, Global Research and Development
| 2020 | 720,000 | 562,500 | 1,999,987 | 0 | 828,576 | 0 | 179,270 | 4,290,333 | ||||||||||||||||||||||||||||||||||||
| 2019 | 720,000 | 837,500 | 1,899,988 | 0 | 734,832 | 0 | 185,405 | 4,377,725 | |||||||||||||||||||||||||||||||||||||
| 2018 | 720,000 | 650,000 | 2,321,392 | 900,003 | 1,296,144 | 0 | 87,492 | 5,975,031 | |||||||||||||||||||||||||||||||||||||
Brendan O’Grady Executive Vice President, North America Commercial
| 2020 | 776,923 | 0 | 2,299,991 | 0 | 991,198 | 0 | 58,171 | 4,126,283 | ||||||||||||||||||||||||||||||||||||
| 2019 | 676,923 | 20,500 | 1,899,988 | 0 | 800,868 | 0 | 64,011 | 3,462,290 | |||||||||||||||||||||||||||||||||||||
Eric Drapé Executive Vice President, Global Operations | 2020 | 708,010 | 0 | 1,799,994 | 0 | 859,028 | 0 | 481,569 | 3,848,601 | ||||||||||||||||||||||||||||||||||||
Salary
| (1) |
|
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 61
Executive Compensation
Bonus
| (2) | The 2020 amount reflected in the table above |
Stock Awards
| (3) | The amounts shown in the Stock Awards column represent the aggregate grant date fair value of the |
The PSUs granted as part of the executive officer annual grants have a three-year performance period and vest in full on the third anniversary of the date of grant. The RSUs granted as part of the executive officer annual grants vest in equal installments on the second, third and fourth anniversaries of the date of grant. For more information on these and other share awards granted during 2017, see the table entitled “2017 Grants of Plan-Based Awards” and related narrative and footnotes.
Under the employment agreement with Mr. Schultz, the Company made twosign-on grants of PSUs, one of which has a three-year performance period and thereafter vests in equal installments generally on the third, fourth, and fifth anniversaries of the date of grant, and the other of which has a five year performance period and thereafter vests in full on the fifth anniversary of the date of grant. In addition, under the employment agreement with Mr. Schultz, the Company made asign-on grant of RSUs that vest in equal installments on the third, fourth and fifth anniversaries of the date of grant.
The grant date fair value of PSUs displayedincluded above is determined based upon achievement of performance at the “target” level, which is the probable outcome of the performance metrics associated with each award of PSUs. If performance were to be achieved at “maximum” level, the grant date fair value of the 2020 PSU awards as of the respective grant dates would have been as follows: Mr. Schultz: five year PSUs—$10,889,293; three year PSUs—$9,105,295; annual PSUs—$3,999,996;$16,799,953; Mr. McClellan: NA; Dr. de Notaristefani: $1,733,315;Kalif: $2,039,983; Dr. Fridriksdottir: $999,957;$2,399,982; Mr. Sabag: $1,066,621;O’Grady: $2,760,002; and Mr. Vigodman: NA; Dr. Peterburg: $2,999,983; Mr. Desheh: $1,333,332; Dr. Koremans: $1,666,651;Drapé: $2,159,993.
See “Compensation Discussion and Dr. Hayden: $1,333,332. These valuesAnalysis—IV. Components of Our Compensation Program— Shareholder-Approved Amendment to CEO Terms of Office and Employment” for details regarding the valuesshareholder approved change in the table do not include the impact of shares that will be forfeited upon the conclusion of the notice period currently in effect for applicable former NEOs.CEO annual equity grant date fair value.
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 63 | |
Executive Compensation
Options
| (4) | The amounts shown above in the Option Awards column represent the aggregate grant date fair value of share options computed in accordance with Topic 718. Valuations of options were determined using the Black-Scholes option pricing model. For information regarding assumptions, factors and methodologies used in our computations pursuant to Topic 718, see note 14c. to our consolidated financial statements in our Annual Report on Form10-K for the year ended December 31, |
Non-Equity Incentive Awards
| (5) | The amounts shown in theNon-Equity Incentive Plan Compensation column are comprised of amounts paid in respect of the executive officer annual cash incentive plan, as determined by the HR and Compensation Committee and the Board in accordance with the plan and the awards |
62 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Executive Compensation
The amount reported for Dr. Fridriksdottir was paid in respect of a performance-based cash award granted when she was an employee of Actavis Generics prior to its acquisition by Teva in 2016. In conjunction with the Actavis Generics acquisition, Teva assumed the obligation to pay the cash incentive based on Actavis Generics shareholder return performance metrics.
The Company paid the amounts reported in 2016 and 2015 for Messrs. Vigodman and Desheh and Dr. Hayden in Israeli shekels. The 2016 U.S. dollar amounts in the table above were converted from Israeli shekels using a 2016 annual average exchange rate of 3.84 shekels per U.S. dollar, and the 2015 U.S. dollar amounts were converted using a 2015 annual average exchange rate of 3.89 shekelsaverage exchange rate of 0.88 euros per U.S. dollar.
All Other Compensation
| (6) |
Name
|
Defined (a)
|
Automobile (b)
|
Life and (c)
|
Housing (d)
|
Tax (e)
|
Study (f)
|
Termination (g)
|
Other (h)
|
Total ($)
| |||||||||||||||||||||||||||
Kåre Schultz
|
|
52,922
|
|
|
15,232
|
|
|
0
|
|
|
41,393
|
|
|
201,339
|
|
|
0
|
|
|
0
|
|
|
153,705
|
|
|
464,591
|
| |||||||||
Michael McClellan
|
|
46,114
|
|
|
13,748
|
|
|
1,080
|
|
|
99,311
|
|
|
39,326
|
|
|
0
|
|
|
0
|
|
|
0
|
|
|
199,579
|
| |||||||||
Dr. Carlo de Notaristefani
|
|
154,641
|
|
|
30,330
|
|
|
1,080
|
|
|
0
|
|
|
0
|
|
|
0
|
|
|
0
|
|
|
3,500
|
|
|
189,551
|
| |||||||||
Dr. Hafrun Fridriksdottir
|
|
54,104
|
|
|
20,308
|
|
|
1,080
|
|
|
0
|
|
|
0
|
|
|
0
|
|
|
0
|
|
|
2,000
|
|
|
77,492
|
| |||||||||
Mark Sabag
|
|
98,058
|
|
|
42,443
|
|
|
103
|
|
|
44,434
|
|
|
82,801
|
|
|
45,348
|
|
|
0
|
|
|
3,921
|
|
|
317,108
|
| |||||||||
Erez Vigodman
|
|
210,953
|
|
|
66,135
|
|
|
0
|
|
|
0
|
|
|
33,761
|
|
|
103,403
|
|
|
1,038,539
|
|
|
2,913
|
|
|
1,455,704
|
| |||||||||
Dr. Yitzhak Peterburg
|
|
235,237
|
|
|
91,024
|
|
|
0
|
|
|
0
|
|
|
40,896
|
|
|
111,451
|
|
|
253,620
|
|
|
4,843
|
|
|
737,071
|
| |||||||||
Eyal Desheh
|
|
131,460
|
|
|
48,122
|
|
|
0
|
|
|
0
|
|
|
43,261
|
|
|
62,357
|
|
|
1,080,471
|
|
|
3,430
|
|
|
1,369,101
|
| |||||||||
Dr. Rob Koremans
|
|
52,588
|
|
|
17,673
|
|
|
0
|
|
|
54,210
|
|
|
21,585
|
|
|
0
|
|
|
1,641,994
|
|
|
19,925
|
|
|
1,807,975
|
| |||||||||
Dr. Michael Hayden
|
|
160,230
|
|
|
48,118
|
|
|
39,255
|
|
|
241,742
|
|
|
219,565
|
|
|
80,344
|
|
|
359,702
|
|
|
3,581
|
|
|
1,152,537
|
| |||||||||
Name | Defined (a) | Automobile (b) | Housing (c) | Tax (d) | Other (e) | Total ($) | ||||||||||||||||||||||||
Kåre Schultz (*) | 318,538 | 89,492 | 214,183 | 43,307 | 3,108 | 668,628 | ||||||||||||||||||||||||
Eli Kalif (*) | 110,863 | 55,688 | — | 42,445 | 56,965 | 265,961 | ||||||||||||||||||||||||
Dr. Hafrun Fridriksdottir | 144,751 | 24,000 | — | 4,670 | 5,849 | 179,270 | ||||||||||||||||||||||||
Brendan O’Grady | 31,950 | 24,000 | — | — | 2,221 | 58,171 | ||||||||||||||||||||||||
Eric Drapé (*) | 172,129 | 46,997 | 117,234 | 97,997 | 47,212 | 481,569 | ||||||||||||||||||||||||
| (*) | The U.S. dollar amounts in the table above were converted from local currency, where needed, using the relevant 2020 monthly average exchange rates of 3.25 to 3.59 Israeli shekels per U.S. dollar and 0.82 to 0.92 euros per U.S. dollar. |
| (a) | Amounts disclosed in this column reflect Company contributions and/or payments related totax-qualified andnon-qualified retirement plans and Israeli |
| (b) | Amounts disclosed in this column reflect automobile allowances, participation in the Company’s car lease program, or use of a Company car and/or reimbursement ofnon-business automobile expenses. |
| (c) | Amounts disclosed in this column reflect |
Amounts disclosed in this column reflect taxgross-ups paid to our NEOs as follows:Mr. Schultz—gross-ups are provided for the income associated with accommodation in Israel, travel costs associated with travel allowance, |
Amounts disclosed in this column reflect |
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 63
Executive Compensation
The U.S. dollar amounts in the table above were converted from local currency using the relevant 2017 monthly average exchange rates of 3.50 to 3.82 Israeli shekels per U.S. dollar and 0.84 to 0.94 euros per U.S. dollar.
Employment Agreements
We have entered into employment agreements with all of our current and former NEOs that provide for, among other things, the term of employment, the position and duties, the compensation and benefits payable during the term
| 64 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Executive Compensation
of the agreement and certain restrictive covenants. The agreements also set forth the terms in the event that the NEO’s employment is terminated under various conditions. The material provisions pertaining to termination of employment of the NEOs are set forth below under “—20172020 Potential Payments Upon Termination or Change in Control.”
Kåre Schultz
On September 7, 2017, we entered into an employment agreement with Mr. Schultz to serve as our PresidentCEO which was amended on June 9, 2020. The employment agreement provides for an employment term of six years, subject to automatic renewal for subsequent one-year periods (or until the second anniversary following a change in control of the Company, if later than the otherwise applicable term end date) until a notice of non-renewal is provided or other termination circumstances occur.
Under the employment agreement, Mr. Schultz receives an annual base salary of $2 million, a performance-based target annual incentive opportunity equal to 140% of his annual base salary (and a maximum opportunity of 200% of his annual base salary) and CEO. Heannual long-term equity incentives with a total target grant date fair value of $10 million with vesting terms similar to other senior executive officers, 70% of which are performance-based. Mr. Schultz is eligible for benefit plans provided to similarly situated executive officers, including medical, dental, group life and other programs, pension and severance contributions as required underpursuant to Israeli law, relocation benefits in accordance with our policy, housing reimbursement up to 40,000 Israeli shekels per month ($11,11011,628 using a 20172020 average monthly exchange rate of 3.603.44 shekels per U.S. dollar) and personal travel reimbursement up to $100,000 per year. We agreed to provideUnder the agreement, Mr. Schultz is also provided with a company car. For a summary of the material terms of Mr. Schultz’s employment
The agreement see “Compensation Discussion and Analysis—IV. Components of Our Compensation Program—Leadership Transitions—Appointment of Mr. Kåre Schultz as President and CEO” above. Mr. Schultz agreed toalso contains noncompetition (except in the event of expiration of his term) and nonsolicitation covenants for 24 months andafter the term of the agreement, a nondisparagement covenant for 10 years after termination. Mr. Schultz also agreed tothe term of the agreement, and an assignment of inventions.
Michael McClellanEli Kalif
Effective as ofOn November 27, 2017,6, 2019, we entered into an employment agreement with Mr. McClellan.Kalif. The agreement provides that Mr. McClellanKalif will be employed as Executive Vice President CFO. Heand CFO, until his death, disability, termination with or without cause or resignation with or without good reason. The agreement provides for an initial annual base salary of 2,343,200 Israeli shekels (approximately $681,163 using a 2020 average monthly exchange rate of 3.44 shekels per U.S. dollar).
Mr. Kalif is eligible to be considered for an annual cash incentive with a target of 100% of his then-current base salary, and for equity-based awards under our equity compensation plan. Under the agreement, Mr. Kalif is also provided with a company or leased car (grossed-up for applicable taxes), certain pension and severance fund contributions pursuant to Israeli law (by both the Company and Mr. Kalif), and group life insurance and other benefits customary for executives in Israel.
In addition, Mr. Kalif received a sign-on equity award in February 2020 in the form of restricted stock units with a grant date fair value of $250,000 in consideration of certain equity grants with Mr. Kalif’s prior employer that were forfeited upon his resignation. These RSUs will vest in three equal installments on the second, third, and fourth anniversaries of the grant date, subject to his continued employment through the applicable vesting dates.
The agreement also contains noncompetition and nonsolicitation covenants for 6 months after the term of the agreement and nondisclosure and nondisparagement covenants and assignment of inventions.
Dr. Hafrun Fridriksdottir
On June 18, 2017, we entered into an executive employment agreement with Dr. Fridriksdottir. The agreement provides that Dr. Fridriksdottir will serve in a senior R&D position until her death, disability,
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 65 | |
Executive Compensation
termination with or without cause or resignation with or without good reason. The agreement provides for an initial annual base salary of $720,000.
Dr. Fridriksdottir is eligible to be considered for an annual cash incentive and for equity-based awards under our equity compensation plan. She is eligible for benefit plans provided to similarly situated executive officers, including medical, disability, dental, life, 401(k) plan, deferred compensation and other programs. In conjunctionUnder the agreement, Dr. Fridriksdottir is also provided with Mr. McClellan’s relocations to the Netherlands and then to Israel, he will be entitled to relocation benefits in accordance with the terms of our relocation policy. While he is based in the Netherlands, he is entitled to a housing allowance of up to€3,250 per month ($3,663 using a 2017 average monthly exchange rate of 0.89 euros per U.S. dollar). For a summary of the material terms of Mr. McClellan’s employment agreement, see “Compensation Discussion and Analysis—IV. Components of Our Compensation Program—Leadership Transitions—Appointment of Mr. Michael McClellan as CFO; Previous Appointment as Interim CFO” above.car allowance.
The agreement also contains noncompetition and nonsolicitation covenants during and for 12 months after the term of the agreement and nondisclosure and nondisparagement covenants and assignment of inventions.
Dr. Carlo de NotaristefaniBrendan O’Grady
On AugustMay 6, 2012,2018, we entered into an executive employment agreement with Dr. de Notaristefani which was amended and restated on February 7, 2018.Mr. O’Grady. The agreement provides that Dr. de NotaristefaniMr. O’Grady will serve in a senior global operations position,as Executive Vice President, North America Commercial until his death, disability, termination with or without cause or resignation with or without good reason. The agreement providedprovides for an initial annual base salary of $836,400.$600,000.
64 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Executive Compensation
Dr. de NotaristefaniMr. O’Grady is eligible to participate in the Company’sbe considered for an annual cash incentive plan with a target of 100% of his then currentthen-current base salary, and for equity-based awards under our equity compensation plan. He is eligible for benefit plans provided to similarly situated executive officers, including medical, disability, dental, life, 401(k) plan, deferred compensation and other programs. We agreed to furnishUnder the agreement, Mr. O’Grady is also provided with a car or a car allowance. In May 2017, we granted Dr. de Notaristefani 30,875 RSUs due to his significance and key role during a critical transition period for us and the importance of securing his services. The RSUs will vest in May 2019.
The agreement also contains noncompetition and nonsolicitation covenants during and for 12 months after the term of the agreement and nondisclosure and nondisparagement covenants and assignment of inventions.
Dr. Hafrun FridriksdottirEric Drapé
On June 18, 2017,March 12, 2020, we entered into an employment agreement with Dr. Fridriksdottir. The agreement provides that Dr. Fridriksdottir will serve in a senior R&D position. She is eligible for benefit plans provided to similarly situated executive officers, including medical, disability, dental, life, 401(k) plan, deferred compensation and other programs. We agreed to provide a car allowance. For a summary of the material terms of Dr. Fridriksdottir’s employment agreement, see “Compensation Discussion and Analysis—IV. Components of Our Compensation Program—Leadership Transitions—Appointment of Dr. Hafrun Fridriksdottir as Executive Vice President, Global R&D” above.
The agreement also contains noncompetition and nonsolicitation covenants during and for 12 months after the term of the agreement and nondisclosure and nondisparagement covenants and assignment of inventions.
Mark Sabag
On December 22, 2013, we entered into an employment agreement with Mr. Sabag.Drapé. The agreement provides that Mr. SabagDrapé will serve as Group Executive Vice President, Human Resources until his death, disability, aged retirement, termination with or without cause or resignation with or without good reason. The agreement provided for an initial base monthly salary of 126,500 Israeli shekels (approximately $35,134 using a 2017 average monthly exchange rate of 3.60 shekels per U.S. dollar). Mr. Sabag is eligible to be considered for an annual cash incentive and for equity-based awards under our equity compensation plan. We agreed to provide Mr. Sabag with a company or leased car andgrossed-up for applicable taxes. We also agreed to provide certain pension and severance fund contributions required in Israel, and group life insurance and other benefits customary for executives in Israel. Mr. Sabag is also eligible for reimbursement of rent up to $3,000 per month, and utilities,grossed-up for applicable taxes. The agreement also contains provisions covering Mr. Sabag’s contributions to a choice of a pension fund, managers’ insurance fund or provident fund.
Mr. Sabag also agreed to a noncompetition covenant during and for 12 months after termination, nondisclosure and nondisparagement covenants and an assignment of inventions.
Erez Vigodman
Effective as of February 11, 2014, we entered into an employment agreement with Mr. Vigodman. The agreement provided that Mr. Vigodman would serve as President and CEOTeva Global Operations until his death, disability, termination with or without cause or resignation with or without good reason. The agreement providedprovides for an initial annual base salary in the amount of Israeli shekels that620,500 euros (approximately $705,114 using a 2020 average monthly exchange rate of 0.88 euros per U.S. dollar).
Mr. Drapé is equivalenteligible to $1,350,000, adjusted according to increases in the consumer price index. Mr. Vigodman was eligiblebe considered for an annual cash incentive with a target of 100% of his then-current base salary, and for equity-based awards under our equity compensation planplan. Mr. Drapé is eligible for benefit plans provided to similarly situated executive officers, including medical, dental, group life and other programs, and pension and severance contributions pursuant to Israeli law, of which the pension portion and any supplement thereof is provided to Mr. Drapé as decided by the Compensation Committee and the Board and subjectreimbursement of an amount equal to the required monthly French contribution, to be paid by Mr. Drapé to French social security to enable continued coverage. Under the agreement, Mr. Drapé is also provided with a company or leased car (grossed-up for applicable framework approved by shareholders. taxes). In conjunction with Mr. Drapé’s relocation to Israel, he is entitled to relocation benefits in accordance with the terms of our relocation policy. He is entitled to a housing allowance of up to 25,000 Israeli shekels per month ($7,267 using a 2020 average monthly exchange rate of 3.44 shekels per U.S. dollar).
The agreement also contains noncompetition and nonsolicitation covenants for 6 months after the term of the agreement and nondisclosure and nondisparagement covenants and assignment of inventions.
2020 Pay Ratio
We alsohave estimated the compensation of the 2020 median employee to be $71,434. The annual total compensation of our CEO was $15,724,518. The ratio of the annual total compensation of our CEO to that of the annual total compensation of our median employee was 220 to 1.
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 65
| 66 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Executive Compensation
agreed to provide certain pension and severance fund contributions required in Israel, medical, dental, group life insurance and other benefits customary for senior executives in Israel. The agreement also contains provisions covering Mr. Vigodman’s contributions to a choice of a pension fund, managers’ insurance fund or provident fund. We agreed to provide Mr. Vigodman with a company car andgrossed-up for applicable taxes.
Mr. Vigodman also agreed to a noncompetition covenant during and for 12 months after the term of the agreement, a nondisparagement covenant for 10 years, a nondisclosure covenant and an assignment of inventions.
Dr. Yitzhak Peterburg
Effective as of February 6, 2017, we entered into an employment agreement with Dr. Peterburg. The agreement provided that Dr. Peterburg would serve as Interim President and CEO until his death, disability, termination with or without cause or resignation with or without good reason. The agreement provided for an initial base monthly salary of 488,250 Israeli shekels (approximately $135,608 using a 2017 average monthly exchange rate of 3.60 shekels per U.S. dollar), adjusted according to increases in the consumer price index. From the appointment of Dr. Peterburg as Interim President and CEO and for as long as he continued to serve in such position, Dr. Peterburg was not entitled to any payments in his capacity as a member of the Board or any committee thereof. Dr. Peterburg was eligible for apro-rata annual cash incentive in 2017. Dr. Peterburg received an equity grant of $4.5 million, comprised of 1/3 options, 1/3 RSUs and 1/3 PSUs. The options and RSUs will vest in three equal installments on the second, third and fourth anniversaries of the grant date and the PSUs will have a cliff vesting on the third anniversary of the grant date subject to meeting the PSU performance goals and in accordance with the formula approved by the Committee and the Board. We agreed to furnish a car andgrossed-up for applicable taxes and to provide certain pension and severance fund contributions required in Israel, medical, dental, group life insurance and other benefits customary for senior executives in Israel. The agreement also contains provisions covering Dr. Peterburg’s contributions to a choice of a pension fund, managers’ insurance fund or provident fund.
Dr. Peterburg also agreed to a noncompetition covenant during and for 12 months after termination, a nondisparagement covenant for 10 years, a nondisclosure covenant and an assignment of inventions.
Eyal Desheh
Effective as of April 28, 2008, we entered into an employment agreement (as subsequently amended) with Mr. Desheh. The agreement provided that Mr. Desheh will serve as CFO until his death, disability, aged retirement, termination with or without cause or resignation with or without good reason. The agreement provided for an initial base monthly salary of 110,000 Israeli shekels (approximately $30,552 using a 2017 average monthly exchange rate of 3.60 shekels per U.S. dollar). Mr. Desheh was eligible for an annual cash incentive and for equity-based awards under our equity compensation plan. We agreed to provide Mr. Desheh with a company or leased car andgrossed-up for applicable taxes. We also agreed to provide certain pension and severance fund contributions required in Israel, and group life insurance and other benefits customary for executives in Israel. The agreement also contains provisions covering Mr. Desheh’s contributions to a choice of a pension fund, managers’ insurance fund or provident fund.
Mr. Desheh also agreed to a noncompetition covenant during and for 12 months after termination, nondisclosure and nondisparagement covenants and an assignment of inventions.
Dr. Rob Koremans
Effective as of March 1, 2012, we entered into an employment agreement (as subsequently amended) with Dr. Rob Koremans. The agreement provided that Dr. Koremans would serve as Teva Pharmaceuticals Europe President and CEO for an indefinite period of time, subject to termination by Dr. Koremans or the
66 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Executive Compensation
Company. The agreement provided for an initial fixed gross annual base salary of€550,000 (approximately $619,896 using a 2017 average monthly exchange rate of 0.89 euro per U.S. dollar). Dr. Koremans was eligible to be considered for an annual cash incentive and for the long term incentive plan. We provided Dr. Koremans with the right to use a Company-leased apartment for which the lease value would not exceed€4,200 per month, until March 31, 2018 (approximately $4,734 using a 2017 average monthly exchange rate of 0.89 euro per U.S. dollar) and a company car for which the annualall-in costs would not exceed an amount of€33,000 (approximately $37,194 using a 2017 average monthly exchange rate of 0.89 euro per U.S. dollar). We also agreed to provide certain group medical, life and disability insurance.
Generally, Dr. Koremans also agreed to noncompetition and nonsolicitation covenants during and for 12 months after termination. If he breaches his obligations, he owes us a penalty of€100,000 and€5,000 for each day that such breach continues (approximately $112,708 and $5,635, respectively, using a 2017 average monthly exchange rate of 0.89 euro per U.S. dollar).
Dr. Michael Hayden
On May 8, 2012, we entered into an employment agreement with Dr. Michael Hayden which was amended and restated on May 22, 2015. The agreement provided that Dr. Hayden would serve as President of R&D and Chief Scientific Officer and will continue on anat-will basis. The agreement provided for an initial base annual salary of $1,050,000. Dr. Hayden was eligible to participate in the Company’s annual cash incentive plan and to be considered for equity awards under the long term incentive plan. We also agreed to be responsible for the costs of existing pension coverage of up to $40,000,grossed-up for taxes, and the balance between such amount and the amount required by Israeli law is contributed to a certain pension fund of his choice. We also agreed to provide certain medical, dental, group life insurance, and other benefits. Dr. Hayden is also entitled to certain benefits associated with his relocation to Israel.
Dr. Hayden also agreed to a noncompetition covenant during and for 12 months after termination, nondisclosure and nondisparagement covenants and an assignment of inventions.
2017 Pay Ratio
Under the Dodd-Frank Wall Street Reform and Consumer Protection Act, we are required to disclose the median of the annual total compensation of our employees, the annual total compensation of our principal executive officer, President and CEO Mr. Kåre Schultz, and the ratio of these two amounts.
We have estimated the median of the 2017 annual total compensation of our employees, excluding Mr. Schultz, to be $64,081. The annualized total compensation of our President and CEO, who was hired in 2017, was $19,374,347. The ratio of the annualized total compensation of our President and CEO to the estimated median of the annual total compensation of our employees was 302 to 1. We believe this pay ratio is a reasonable estimate calculated in a manner consistent with SEC rules. We note that a substantial portion of our President and CEO’s total compensation for 2017 was thesign-on equity awards he received in accordance with his employment agreement, which had a grant date fair value of approximately $10.2 million. Excluding thesign-on equity awards, the ratio would have been 143 to 1.
The following paragraphs provide important context related to our employee population and describe the methodology and the material assumptions, adjustments, and estimates that we used to calculate this ratio.
Teva is a global company, with complex operations worldwide and with many of its executive officers and a majority of its employees located outside of Israel, the country in which our headquarters office is located.
As of November 1, 2017, Teva’s workforce consisted of approximately 52,419 full-time and part-time employees, including hourly employees, who worked for our parent company and consolidated subsidiaries. Approximately 45% of these employees are located in Europe, approximately 17% are located
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 67
Executive Compensation
in the U.S., approximately 12% are located in Israel, and approximately 26% are located throughout the rest of the world. Approximately 50,441 individuals are full-time employees, with the remainder employed on a part-time basis.
In determining the employee population to be used to calculate the compensation of the median employee, we included employees in all countries except for 424 employees in Venezuela, who represented less than 5% of our total employees, as permitted under the applicable SEC de minimis rule. As a result, the employee population that we used for purposes of determining the compensation of our median employee was 51,995 employees.
We selected November 1, 2017, which is within the last three months of 2017,December 31, 2020, as the date upon which we would identify the “median employee,” because it enabled us to make such identification in a reasonably efficientemployee” and economical manner, and it was also the date that our new CEO commenced employment.
We included employees from all of our full-time, part-time, and temporary employees globally, but excluded our President and CEO. We annualized the compensation of approximately 2,562 full-time and part-time employees who were hired in 2017 but did not work for us for the entire fiscal year.relevant countries. Earnings of our employees outside the U.S. were converted to U.S. dollars using the average December currency exchange rates used for organizational planning purposes, which consider historic and forecasted rates as well as other factors. We did not make any cost of living adjustments.rates.
To identify the “median employee,” we utilized the annualized 20172020 base salary and target annual cash incentive for our consistently applied compensation measure because we believe that this measure reasonably reflects the annual compensation of our employees. We do not grant equity to a large percentage of our employee population, so using base salary plus target annual incentive is representative.
Using this measure, we identified aour “median employee” who is a full-time, salaried employee located in Israel. Initially, a different employee had been identified, but in the process of determining that employee’s total compensation in accordance with applicable SEC rules, we recognized that there were anomalous elements in that employee’s compensation which we believe did not reasonably reflect the annual compensation of our employees generally. Consequently, we identified an employee whose amount for the consistently applied compensation measure was very close to the initial employee, but who did not have such unusual elements. Once we identified this median employee, weWe totaled all of the elements of the employee’s compensation for 20172020 in the same manner as the CEO and in accordance with theSEC Summary Compensation Table disclosure requirements, of the applicable SEC rules and converted the amounts from Israeli shekels to U.S. dollars using the relevant monthly average currency exchange rate of 3.50 to 3.82 shekels per U.S. dollar. Thiswhich resulted in an annual total compensation of $64,081,$71,434, of which $29,159$29,956 is base salary, $5,476 is non-equity incentive compensation, and $34,922$36,002 is comprised of Company contributions to a pension fund, as is required by Israeli law, and other compensation such as overtime pay travel and other cash allowances, and Company contributions to a study fund, as is common practice for Israel-based employees of the Company.
With respect to the annual total compensation of our President and CEO, we adjusted the amount reported in the “Total” column of our 2017 Summary Compensation Table included in this Proxy Statement, by annualizing his base salary and certain components of “all other compensation” to account for the fact that he only commenced employment with us on November 1, 2017, resulting in an adjusted total amount of $19,374,347. As indicated above, we note that a substantial portion of the total compensation of our newly-hired President and CEO for 2017 was thesign-on equity awards he received in accordance with his employment agreement, which had a grant date fair value of approximately $10.2 million.
Because the SEC rules for identifying the median of the annual total compensation of our employees and calculating the pay ratio based on that employee’s annual total compensation allow companies to adopt a variety of methodologies, to apply certain exclusions, and to make reasonable estimates and assumptions
68 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Executive Compensation
that reflect their employee populations and compensation practices, the pay ratio reported by other companies may not be comparable to the pay ratio for our Company, as other companies have headquarters offices in different countries, have different employee populations and compensation practices and may utilize different methodologies, exclusions, estimates and assumptions in calculating their pay ratios.
2017 Grants of Plan-Based Awards
Estimated Future Payouts
| Estimated Future Payouts
| |||||||||||||||||||||||||||||||||||||||||||||
Name
| Approval
| Grant
| Award Type
| Threshold
| Target
| Maximum
| Threshold
| Target
| Maximum
| All (#) (3)
| All Other of
| Exercise Base
| Grant Fair of Share
| |||||||||||||||||||||||||||||||||
Kåre Schultz |
9/6/2017 |
11/1/2017 |
Annual Incentive |
|
0 |
|
|
2,800,000 |
|
|
4,000,000 |
| ||||||||||||||||||||||||||||||||||
9/6/2017 |
11/3/2017 |
PSU (5) |
|
0 |
|
|
649,914 |
|
|
1,949,742 |
|
|
3,035,098 |
| ||||||||||||||||||||||||||||||||
9/6/2017 |
11/3/2017 |
PSU (5) | 0 | 751,504 | 2,254,512 | 3,629,764 | ||||||||||||||||||||||||||||||||||||||||
9/6/2017 |
11/3/2017 |
RSU (5) |
|
349,163 |
|
|
3,564,954 |
| ||||||||||||||||||||||||||||||||||||||
9/6/2017 |
11/3/2017 |
PSU |
|
0 |
|
|
212,314 |
|
|
424,628 |
|
|
1,999,998 |
| ||||||||||||||||||||||||||||||||
9/6/2017 |
11/3/2017 |
RSU |
|
190,839 |
|
|
1,999,993 |
| ||||||||||||||||||||||||||||||||||||||
9/6/2017 |
11/3/2017 |
Options |
|
591,719 |
|
|
11.40 |
|
|
2,000,009 |
| |||||||||||||||||||||||||||||||||||
Michael
McClellan |
7/12/2017 |
7/12/2017 |
Annual Incentive |
|
0 |
|
|
219,519 |
|
|
439,038 |
| ||||||||||||||||||||||||||||||||||
2/28/2017 |
3/3/2017 |
RSU |
|
4,197 |
|
|
132,331 |
| ||||||||||||||||||||||||||||||||||||||
2/28/2017 |
3/3/2017 |
Options |
|
22,505 |
|
|
34.70 |
|
|
132,329 |
| |||||||||||||||||||||||||||||||||||
9/18/2017 |
9/18/2017 |
RSU |
|
4,091 |
|
|
66,929 |
| ||||||||||||||||||||||||||||||||||||||
| 9/18/2017 | 9/18/2017 | Options | 12,341 | 16.99 | 63,186 | |||||||||||||||||||||||||||||||||||||||||
Dr. Carlo de
Notaristefani |
3/31/2017 |
3/31/2017 |
Annual Incentive |
|
0 |
|
|
836,400 |
|
|
1,672,800 |
| ||||||||||||||||||||||||||||||||||
2/7/2017 |
2/14/2017 |
PSU |
|
0 |
|
|
30,941 |
|
|
61,882 |
|
|
866,657 |
| ||||||||||||||||||||||||||||||||
2/7/2017 |
2/14/2017 |
RSU |
|
27,840 |
|
|
866,659 |
| ||||||||||||||||||||||||||||||||||||||
2/7/2017 |
2/14/2017 |
Options |
|
147,396 |
|
|
34.90 |
|
|
866,688 |
| |||||||||||||||||||||||||||||||||||
3/31/2017 |
5/18/2017 |
RSU |
|
30,875 |
|
|
836,404 |
| ||||||||||||||||||||||||||||||||||||||
Dr. Hafrun
Fridriksdottir |
3/31/2017 |
3/31/2017 |
Annual Incentive |
|
0 |
|
|
630,577 |
|
|
1,261,154 |
| ||||||||||||||||||||||||||||||||||
2/7/2017 |
2/14/2017 |
PSU |
|
0 |
|
|
17,850 |
|
|
35,700 |
|
|
499,979 |
| ||||||||||||||||||||||||||||||||
2/7/2017 |
2/14/2017 |
RSU |
|
16,061 |
|
|
499,979 |
| ||||||||||||||||||||||||||||||||||||||
2/7/2017 |
2/14/2017 |
Options |
|
85,042 |
|
|
34.90 |
|
|
500,047 |
| |||||||||||||||||||||||||||||||||||
Mark Sabag |
3/31/2017 |
3/31/2017 |
Annual Incentive |
|
0 |
|
|
604,637 |
|
|
1,209,274 |
| ||||||||||||||||||||||||||||||||||
2/7/2017 |
2/14/2017 |
PSU |
|
0 |
|
|
19,040 |
|
|
38,080 |
|
|
533,310 |
| ||||||||||||||||||||||||||||||||
2/7/2017 |
2/14/2017 |
RSU |
|
17,132 |
|
|
533,319 |
| ||||||||||||||||||||||||||||||||||||||
2/7/2017 |
2/14/2017 |
Options |
|
90,710 |
|
|
34.90 |
|
|
533,375 |
| |||||||||||||||||||||||||||||||||||
Dr. Yitzhak
Peterburg |
3/31/2017 |
3/31/2017 |
Annual Incentive |
|
0 |
|
|
1,679,650 |
|
|
2,351,510 |
| ||||||||||||||||||||||||||||||||||
2/8/2017 |
2/14/2017 |
PSU |
|
0 |
|
|
53,552 |
|
|
107,104 |
|
|
1,499,992 |
| ||||||||||||||||||||||||||||||||
2/8/2017 |
2/14/2017 |
RSU |
|
48,185 |
|
|
1,499,999 |
| ||||||||||||||||||||||||||||||||||||||
2/8/2017 |
2/14/2017 |
Share Options |
|
255,104 |
|
|
34.90 |
|
|
1,500,009 |
| |||||||||||||||||||||||||||||||||||
Eyal Desheh |
3/31/2017 |
3/31/2017 |
Annual Incentive |
|
0 |
|
|
408,300 |
|
|
816,600 |
| ||||||||||||||||||||||||||||||||||
2/7/2017 |
2/14/2017 |
PSU |
|
0 |
|
|
23,801 |
|
|
47,602 |
|
|
666,666 |
| ||||||||||||||||||||||||||||||||
2/7/2017 |
2/14/2017 |
RSU |
|
21,415 |
|
|
666,649 |
| ||||||||||||||||||||||||||||||||||||||
2/7/2017 |
2/14/2017 |
Options |
|
113,382 |
|
|
34.90 |
|
|
666,686 |
| |||||||||||||||||||||||||||||||||||
Dr. Rob
Koremans |
3/31/2017 |
3/31/2017 |
Annual Incentive |
|
0 |
|
|
783,215 |
|
|
1,566,430 |
| ||||||||||||||||||||||||||||||||||
2/7/2017 |
2/14/2017 |
PSU |
|
0 |
|
|
29,751 |
|
|
59,502 |
|
|
833,326 |
| ||||||||||||||||||||||||||||||||
2/7/2017 |
2/14/2017 |
RSU |
|
26,769 |
|
|
833,319 |
| ||||||||||||||||||||||||||||||||||||||
2/7/2017 |
2/14/2017 |
Options |
|
141,728 |
|
|
34.90 |
|
|
833,361 |
| |||||||||||||||||||||||||||||||||||
Dr. Michael
Hayden |
3/31/2017 |
3/31/2017 |
Annual Incentive |
|
0 |
|
|
1,071,000 |
|
|
2,142,000 |
| ||||||||||||||||||||||||||||||||||
2/7/2017 |
2/14/2017 |
PSU |
|
0 |
|
|
23,801 |
|
|
47,602 |
|
|
666,666 |
| ||||||||||||||||||||||||||||||||
2/7/2017 |
2/14/2017 |
RSU |
|
21,415 |
|
|
666,649 |
| ||||||||||||||||||||||||||||||||||||||
2/7/2017 |
2/14/2017 |
Options |
|
113,382 |
|
|
34.90 |
|
|
666,686 |
| |||||||||||||||||||||||||||||||||||
Mr. Vigodman did not receive any grants of plan-based awards in 2017. In addition, the annual equity award granted to Mr. McClellan was made prior to his appointment as Interim CFO in July 2017 pursuant to our program fornon-executive officers.
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 69
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 67 | |
Executive Compensation
2020 Grants of Plan-Based Awards
| Estimated Future Payouts Under Non-Equity Incentive Plan Awards (1) | Estimated Future Payouts Under Equity Incentive Plan Awards (2) | |||||||||||||||||||||||||||||||||||||||||||||||||
Name | Approval Date | Grant Date | Award Type | Threshold ($) | Target ($) | Maximum ($) | Threshold (#) | Target (#) | Maximum (#) | All (#) (3) | All Other (#) | Exercise or Base Price of Option Awards ($/Sh) | Grant ($) | |||||||||||||||||||||||||||||||||||||
Kåre Schultz |
|
|
|
|
|
| Annual Incentive | 500,000 | 2,800,000 | 4,000,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||
| 2/11/2020 | 2/28/2020 | PSU |
|
|
|
|
|
|
|
|
| 57,820 | 289,100 | 693,840 |
|
|
|
|
|
|
|
|
| 2,999,991 | |||||||||||||||||||||||||
| 2/11/2020 | 2/28/2020 | RSU |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 260,190 |
|
|
|
|
|
| 2,999,991 | |||||||||||||||||||||
| 6/9/2020 | 6/9/2020 | PSU |
|
|
|
|
|
|
|
|
| 70,361 | 351,802 | 844,325 |
|
|
| �� |
|
|
|
|
|
| 3,999,989 | ||||||||||||||||||||||||
Eli Kalif |
|
|
|
|
|
| Annual Incentive | 170,320 | 681,281 | 1,362,563 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||
| 11/6/2019 | 2/13/2020 | RSU |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 19,888 |
|
|
|
|
|
| 249,992 | |||||||||||||||||||||
| 2/11/2020 | 2/28/2020 | PSU |
|
|
|
|
|
|
|
|
| 16,383 | 81,911 | 196,587 |
|
|
|
|
|
|
|
|
| 849,990 | |||||||||||||||||||||||||
| 2/11/2020 | 2/28/2020 | RSU |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 73,720 |
|
|
|
|
|
| 849,992 | |||||||||||||||||||||
Dr. Hafrun Fridriksdottir |
|
|
|
|
|
| Annual Incentive | 180,000 | 720,000 | 1,440,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||
| 2/11/2020 | 2/28/2020 | PSU |
|
|
|
|
|
|
|
|
| 19,274 | 96,366 | 231,279 |
|
|
|
|
|
|
|
|
| 999,990 | |||||||||||||||||||||||||
| 2/11/2020 | 2/28/2020 | RSU |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 86,730 |
|
|
|
|
|
| 999,997 | |||||||||||||||||||||
Brendan O’Grady |
|
|
|
|
|
| Annual Incentive | 194,231 | 776,923 | 1,553,846 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||
| 2/11/2020 | 2/28/2020 | PSU |
|
|
|
|
|
|
|
|
| 22,165 | 110,822 | 265,973 |
|
|
|
|
|
|
|
|
| 1,150,000 | |||||||||||||||||||||||||
| 2/11/2020 | 2/28/2020 | RSU |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 99,739 |
|
|
|
|
|
| 1,149,991 | |||||||||||||||||||||
Eric Drapé |
|
|
|
|
|
| Annual Incentive | 177,002 | 708,010 | 1,416,020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||
| 2/11/2020 | 2/28/2020 | PSU |
|
|
|
|
|
|
|
|
| 17,346 | 86,730 | 208,152 |
|
|
|
|
|
|
|
|
| 899,997 | |||||||||||||||||||||||||
|
| 2/11/2020 | 2/28/2020 | RSU |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 78,057 |
|
|
|
|
|
| 899,997 | |||||||||||||||||||||
Annual Incentive Plan
| (1) | The amounts disclosed in these columns reflect the threshold, target and maximum annual cash incentive opportunities for |
Mr. McClellan and Dr. Fridriksdottir were eligible for annual incentive award opportunities under the Company’s plan fornon-executive officers in respect ofpro-rated salary earned prior to being appointed executive officers. Pursuant to this plan fornon-executive officers, Mr. McClellan and Dr. Fridriksdottir hadpro-rated target opportunities of $88,769 and $52,615, respectively, and maximumpro-rated incentive opportunities of $177,538 and $105,230, respectively. Because the Company’s financial results were significantly below our original financial targets for the year, no payouts were made for Mr. McClellan and Dr. Fridriksdottir under this incentive plan.
Performance Share Units (PSUs)
| (2) | Amounts disclosed in these columns reflect the potential threshold, target and maximum number of PSUs awarded in |
See “Compensation Discussion and Analysis—IV. Components of Our Compensation Program—Shareholder-Approved Amendment to CEO Terms of Office and Employment” for details regarding the shareholder approved change in CEO annual equity grant date fair value.
Restricted Share Units (RSUs)
| (3) | Amounts disclosed in this column reflect the number of RSUs granted to our NEOs in |
Share Options
| 68 | Teva | |
Sign-On Grants to Mr. Schultz of PSUs and RSUs
70 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Executive Compensation
20172020 Outstanding Equity Awards at FiscalYear-End
Option Awards
|
Stock Awards
| |||||||||||||||||||||||||||||||||||||
Name
| Award
| Grant
| Number of
| Number of (2)
| Option
| Option
| Number
| Market
| Equity
| Equity ($) (6)
| Vesting Schedule (7)
| |||||||||||||||||||||||||||
Kåre Schultz
| Options
| 11/3/2017
|
| 591,719
|
|
| 11.40
|
|
| 11/2/2027
|
| 33% in 2019, 2020 and 2021
| ||||||||||||||||||||||||||
| RSUs
| 11/3/2017
|
| 190,839
|
|
| 3,616,399
|
| 33% in 2019, 2020 and 2021
| ||||||||||||||||||||||||||||||
| 11/3/2017
|
| 349,163
|
|
| 6,616,639
|
| 33% in 2020, 2021 and 2022
| |||||||||||||||||||||||||||||||
| PSUs
| 11/3/2017
|
| 649,914
|
|
| 12,315,870
|
| 33% in 2020, 2021 and 2022, subject to performance
| ||||||||||||||||||||||||||||||
| 11/3/2017
|
| 751,504
|
|
| 14,241,001
|
| 100% in 2022, subject to performance
| |||||||||||||||||||||||||||||||
| 11/3/2017
|
| 212,314
|
|
| 4,023,350
|
| 100% in 2020, subject to performance
| |||||||||||||||||||||||||||||||
Michael McClellan
| Options
| 11/5/2015
|
| 6,962
|
|
| 6,965
|
|
| 60.92
|
|
| 11/4/2025
|
| 25% in 2016, 2017, 2018 and 2019
| |||||||||||||||||||||||
| 3/17/2016
|
| 3,500
|
|
| 10,503
|
|
| 53.50
|
|
| 3/16/2026
|
| 25% in 2017, 2018, 2019 and 2020
| |||||||||||||||||||||||||
| 3/3/2017
|
| 22,505
|
|
| 34.70
|
|
| 3/2/2027
|
| 25% in 2018, 2019, 2020 and 2021
| ||||||||||||||||||||||||||||
| 9/18/2017
|
| 12,341
|
|
| 16.99
|
|
| 9/17/2027
|
| 100% in 2019
| ||||||||||||||||||||||||||||
| RSUs
| 11/5/2015
|
| 1,390
|
|
| 26,341
|
| 25% in 2016, 2017, 2018 and 2019
| ||||||||||||||||||||||||||||||
| 3/17/2016
|
| 1,981
|
|
| 37,540
|
| 25% in 2017, 2018, 2019 and 2020
| |||||||||||||||||||||||||||||||
| 3/3/2017
|
| 4,197
|
|
| 79,533
|
| 25% in 2018, 2019, 2020 and 2021
| |||||||||||||||||||||||||||||||
| 9/18/2017
|
| 4,091
|
|
| 77,524
|
| 100% in 2019
| |||||||||||||||||||||||||||||||
Dr. Carlo de Notaristefani
| Options
| 8/1/2012
|
| 150,003
|
|
| 40.87
|
|
| 7/31/2022
|
| vested
| ||||||||||||||||||||||||||
| 3/12/2014
|
| 65,720
|
|
| 32,861
|
|
| 48.76
|
|
| 3/11/2024
|
| 33% in 2016, 2017 and 2018
| |||||||||||||||||||||||||
| 2/12/2015
|
| 29,792
|
|
| 59,584
|
|
| 57.35
|
|
| 2/11/2025
|
| 33% in 2017, 2018 and 2019
| |||||||||||||||||||||||||
| 2/12/2016
|
| 99,904
|
|
| 55.75
|
|
| 2/11/2026
|
| 33% in 2018, 2019 and 2020
| ||||||||||||||||||||||||||||
| 5/16/2016
|
| 8,346
|
|
| 50.43
|
|
| 5/15/2026
|
| 33% in 2018, 2019 and 2020
| ||||||||||||||||||||||||||||
| 2/14/2017
|
| 147,396
|
|
| 34.90
|
|
| 2/13/2027
|
| 33% in 2019, 2020 and 2021
| ||||||||||||||||||||||||||||
| RSUs
| 2/14/2017
|
| 27,840
|
|
| 527,568
|
| 33% in 2019, 2020 and 2021
| ||||||||||||||||||||||||||||||
| 5/18/2017
|
| 30,875
|
|
| 585,081
|
| 100% in 2019
| |||||||||||||||||||||||||||||||
| PSUs
| 2/12/2016
|
| 19,219
|
|
| 364,200
|
| 100% in 2019, subject to performance
| ||||||||||||||||||||||||||||||
| 5/16/2016
|
| 1,603
|
|
| 30,377
|
| 100% in 2019, subject to performance
| |||||||||||||||||||||||||||||||
| 2/14/2017
|
| 30,941
|
|
| 586,332
|
| 100% in 2020, subject to performance
| |||||||||||||||||||||||||||||||
Dr. Hafrun Fridriksdottir
| Options
| 7/1/2014
|
| 7,603
|
|
| 15,206
|
|
| 48.69
|
|
| 6/30/2024
|
| 33% in 2017, 2018 and 2019
| |||||||||||||||||||||||
| 8/2/2016
|
| 3,997
|
|
| 11,993
|
|
| 52.96
|
|
| 8/1/2026
|
| 25% in 2017, 2018, 2019 and 2020
| |||||||||||||||||||||||||
| 9/9/2016
|
| 1,388
|
|
| 4,165
|
|
| 50.21
|
|
| 9/8/2026
|
| 25% in 2017, 2018, 2019 and 2020
| |||||||||||||||||||||||||
| 11/30/2016
|
| 57,167
|
|
| 37.70
|
|
| 11/29/2026
|
| 25% in 2018, 75% in 2019
| ||||||||||||||||||||||||||||
| 2/14/2017
|
| 85,042
|
|
| 34.90
|
|
| 2/13/2027
|
| 33% in 2019, 2020 and 2021
| ||||||||||||||||||||||||||||
| RSUs
| 5/8/2014
|
| 3,432
|
|
| 65,036
|
| 50% in 2017 and 2018
| ||||||||||||||||||||||||||||||
| 7/1/2014
|
| 9,280
|
|
| 175,856
|
| 33% in 2017, 2018 and 2019
| |||||||||||||||||||||||||||||||
| 3/4/2015
|
| 2,330
|
|
| 44,154
|
| 33% in 2017, 2018 and 2019
| |||||||||||||||||||||||||||||||
| 8/2/2016
|
| 2,242
|
|
| 42,486
|
| 25% in 2017, 2018, 2019 and 2020
| |||||||||||||||||||||||||||||||
| 9/9/2016
|
| 796
|
|
| 15,084
|
| 25% in 2017, 2018, 2019 and 2020
| |||||||||||||||||||||||||||||||
| 11/30/2016
|
| 10,844
|
|
| 205,494
|
| 25% in 2018, 75% in 2019
| |||||||||||||||||||||||||||||||
| 2/14/2017
|
| 16,061
|
|
| 304,356
|
| 33% in 2019, 2020 and 2021
| |||||||||||||||||||||||||||||||
| PSUs
| 2/14/2017
|
| 17,850
|
|
| 338,258
|
| 100% in 2020, subject to performance
| ||||||||||||||||||||||||||||||
|
|
|
| Option Awards | Stock Awards |
| |||||||||||||||||||||||||||||||||
Name | Award Type | Grant Date | Number of Securities Underlying Unexercised Options (#) Exercisable (1) | Number of Securities Underlying Unexercised Options (#) Unexercisable (2) | Option Exercise Price ($) | Option Expiration Date | Number of Shares or Units of Shares That Have Not Vested (#) (3) | Market Value of Shares or Units of Shares That Have Not Vested ($) (4) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) (5) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That have Not Vested ($) (6) | Vesting Schedule (7) | |||||||||||||||||||||||||||
Kåre Schultz | Options | 11/3/2017 | 394,478 | 197,241 | 11.40 | 11/3/2027 |
|
|
|
|
|
|
|
|
|
|
|
| 33% in 2019, 2020 and 2021 | |||||||||||||||||||
|
| 2/9/2018 | 68,494 | 136,988 | 18.61 | 2/9/2028 |
|
|
|
|
|
|
|
|
|
|
|
| 33% in 2020, 2021 and 2022 | |||||||||||||||||||
| RSUs | 11/3/2017 |
|
|
|
|
|
|
|
|
|
|
|
| 63,613 | 613,865 |
|
|
|
|
|
| 33% in 2019, 2020 and 2021 | |||||||||||||||
|
| 11/3/2017 |
|
|
|
|
|
|
|
|
|
|
|
| 232,776 | 2,246,288 |
|
|
|
|
|
| 33% in 2020, 2021 and 2022 | |||||||||||||||
|
| 2/9/2018 |
|
|
|
|
|
|
|
|
|
|
|
| 53,734 | 518,533 |
|
|
|
|
|
| 33% in 2020, 2021 and 2022 | |||||||||||||||
|
| 3/4/2019 |
|
|
|
|
|
|
|
|
|
|
|
| 179,104 | 1,728,354 |
|
|
|
|
|
| 33% in 2021, 2022 and 2023 | |||||||||||||||
|
| 2/28/2020 |
|
|
|
|
|
|
|
|
|
|
|
| 260,190 | 2,510,834 |
|
|
|
|
|
| 25% in 2021, 2022, 2023 and 2024 | |||||||||||||||
| PSUs | 11/3/2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 375,752 | 3,626,007 | 100% in 2022, subject to performance | |||||||||||||||
|
| 2/9/2018 |
|
|
|
|
|
|
|
|
|
|
|
| 226,173 | 2,182,569 |
|
|
|
|
|
| 100% in 2021 | |||||||||||||||
|
| 3/4/2019 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 198,938 | 1,919,752 | 100% in 2022, subject to performance | |||||||||||||||
|
| 2/28/2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 72,275 | 697,454 | 100% in 2023, subject to performance | |||||||||||||||
|
| 6/9/2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 87,951 | 848,727 | 100% in 2023, subject to performance | |||||||||||||||
Eli Kalif | RSUs | 2/13/2020 |
|
|
|
|
|
|
|
|
|
|
|
| 19,888 | 191,919 |
|
|
|
|
|
| 33% in 2022, 2023 and 2024 | |||||||||||||||
|
| 2/28/2020 |
|
|
|
|
|
|
|
|
|
|
|
| 73,720 | 711,398 |
|
|
|
|
|
| 25% in 2021, 2022, 2023 and 2024 | |||||||||||||||
| PSUs | 2/28/2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 20,478 | 197,613 | 100% in 2023, subject to performance | |||||||||||||||
Dr. Hafrun Fridriksdottir | Options | 7/1/2014 | 22,809 |
|
|
| 48.69 | 7/1/2024 |
|
|
|
|
|
|
|
|
|
|
|
| vested | |||||||||||||||||
|
| 8/2/2016 | 15,990 |
|
|
| 52.96 | 8/2/2026 |
|
|
|
|
|
|
|
|
|
|
|
| vested | |||||||||||||||||
|
| 9/9/2016 | 5,553 |
|
|
| 50.21 | 9/9/2026 |
|
|
|
|
|
|
|
|
|
|
|
| vested | |||||||||||||||||
|
| 11/30/2016 | 57,167 |
|
|
| 37.70 | 11/30/2026 |
|
|
|
|
|
|
|
|
|
|
|
| vested | |||||||||||||||||
|
| 2/14/2017 | 56,694 | 28,348 | 34.90 | 2/14/2027 |
|
|
|
|
|
|
|
|
|
|
|
| 33% in 2019, 2020 and 2021 | |||||||||||||||||||
|
| 2/9/2018 | 41,096 | 82,192 | 18.61 | 2/9/2028 |
|
|
|
|
|
|
|
|
|
|
|
| 33% in 2020, 2021 and 2022 | |||||||||||||||||||
| RSUs | 2/14/2017 |
|
|
|
|
|
|
|
|
|
|
|
| 5,355 | 51,676 |
|
|
|
|
|
| 33% in 2019, 2020 and 2021 | |||||||||||||||
|
| 2/9/2018 |
|
|
|
|
|
|
|
|
|
|
|
| 32,241 | 311,126 |
|
|
|
|
|
| 33% in 2020, 2021 and 2022 | |||||||||||||||
|
| 3/4/2019 |
|
|
|
|
|
|
|
|
|
|
|
| 56,716 | 547,309 |
|
|
|
|
|
| 33% in 2021, 2022 and 2023 | |||||||||||||||
|
| 2/28/2020 |
|
|
|
|
|
|
|
|
|
|
|
| 86,730 | 836,945 |
|
|
|
|
|
| 25% in 2021, 2022, 2023 and 2024 | |||||||||||||||
| PSUs | 2/9/2018 |
|
|
|
|
|
|
|
|
|
|
|
| 67,852 | 654,772 |
|
|
|
|
|
| 100% in 2021 | |||||||||||||||
|
| 5/11/2018 |
|
|
|
|
|
|
|
|
|
|
|
| 37,884 | 365,581 |
|
|
|
|
|
| 100% in 2021 | |||||||||||||||
|
| 3/4/2019 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 62,997 | 607,921 | 100% in 2022, subject to performance | |||||||||||||||
|
| 2/28/2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 24,092 | 232,488 | 100% in 2023, subject to performance | |||||||||||||||
Brendan O’Grady | Options | 11/7/2011 | 9,003 |
|
|
| 41.72 | 11/7/2021 |
|
|
|
|
|
|
|
|
|
|
|
| vested | |||||||||||||||||
|
| 12/13/2012 | 12,503 |
|
|
| 38.84 | 12/13/2022 |
|
|
|
|
|
|
|
|
|
|
|
| vested | |||||||||||||||||
|
| 3/12/2014 | 17,502 |
|
|
| 48.76 | 3/12/2024 |
|
|
|
|
|
|
|
|
|
|
|
| vested | |||||||||||||||||
|
| 3/12/2015 | 15,502 |
|
|
| 60.21 | 3/12/2025 |
|
|
|
|
|
|
|
|
|
|
|
| vested | |||||||||||||||||
|
| 3/17/2016 | 25,006 |
|
|
| 53.50 | 3/17/2026 |
|
|
|
|
|
|
|
|
|
|
|
| vested | |||||||||||||||||
|
| 3/3/2017 | 18,750 | 6,251 | 34.70 | 3/3/2027 |
|
|
|
|
|
|
|
|
|
|
|
| 25% in 2018, 2019, 2020 and 2021 | |||||||||||||||||||
|
| 9/18/2017 | 7,485 |
|
|
| 16.99 | 9/18/2027 |
|
|
|
|
|
|
|
|
|
|
|
| vested | |||||||||||||||||
|
| 2/9/2018 | 41,096 | 82,192 | 18.61 | 2/9/2028 |
|
|
|
|
|
|
|
|
|
|
|
| 33% in 2020, 2021 and 2022 | |||||||||||||||||||
| RSUs | 3/3/2017 |
|
|
|
|
|
|
|
|
|
|
|
| 1,166 | 11,252 |
|
|
|
|
|
| 25% in 2018, 2019, 2020 and 2021 | |||||||||||||||
|
| 2/9/2018 |
|
|
|
|
|
|
|
|
|
|
|
| 32,241 | 311,126 |
|
|
|
|
|
| 33% in 2020, 2021 and 2022 | |||||||||||||||
|
| 3/4/2019 |
|
|
|
|
|
|
|
|
|
|
|
| 56,716 | 547,309 |
|
|
|
|
|
| 33% in 2021, 2022 and 2023 | |||||||||||||||
|
| 2/28/2020 |
|
|
|
|
|
|
|
|
|
|
|
| 99,739 | 962,481 |
|
|
|
|
|
| 25% in 2021, 2022, 2023 and 2024 | |||||||||||||||
| PSUs | 2/9/2018 |
|
|
|
|
|
|
|
|
|
|
|
| 67,852 | 654,772 |
|
|
|
|
|
| 100% in 2021 | |||||||||||||||
|
| 3/4/2019 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 62,997 | 607,921 | 100% in 2022, subject to performance | |||||||||||||||
|
|
| 2/28/2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 27,706 | 267,363 | 100% in 2023, subject to performance | |||||||||||||||
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 71
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 69 | |
Executive Compensation
Option Awards
|
Stock Awards
| |||||||||||||||||||||||||||||||||||||
Name
| Award
| Grant
| Number of
| Number of (2)
| Option
| Option
| Number
| Market
| Equity
| Equity ($) (6)
| Vesting Schedule (7)
| |||||||||||||||||||||||||||
Mark Sabag
| Options
| 11/7/2011
|
| 4001
|
|
| 41.72
|
|
| 11/6/2021
|
| vested
| ||||||||||||||||||||||||||
| 2/24/2012
|
| 3,201
|
|
| 44.59
|
|
| 2/23/2022
|
| vested
| ||||||||||||||||||||||||||||
| 12/13/2012
|
| 4,501
|
|
| 38.84
|
|
| 12/12/2022
|
| vested
| ||||||||||||||||||||||||||||
| 2/24/2013
|
| 4,502
|
|
| 38.08
|
|
| 2/23/2023
|
| vested
| ||||||||||||||||||||||||||||
| 11/11/2013
|
| 100,002
|
|
| 37.26
|
|
| 11/10/2023
|
| vested
| ||||||||||||||||||||||||||||
| 3/12/2014
|
| 49,288
|
|
| 24,644
|
|
| 48.76
|
|
| 3/11/2024
|
| 33% in 2016, 2017 and 2018
| |||||||||||||||||||||||||
| 2/12/2015
|
| 22,345
|
|
| 44,690
|
|
| 57.35
|
|
| 2/11/2025
|
| 33% in 2017, 2018 and 2019
| |||||||||||||||||||||||||
| 2/12/2016
|
| 64,940
|
|
| 55.75
|
|
| 2/11/2026
|
| 33% in 2018, 2019 and 2020
| ||||||||||||||||||||||||||||
| 2/14/2017
|
| 90,710
|
|
| 34.90
|
|
| 2/13/2027
|
| 33% in 2019, 2020 and 2021
| ||||||||||||||||||||||||||||
| RSUs
| 2/14/2017
|
| 17,132
|
|
| 324,651
|
| 33% in 2019, 2020 and 2021
| ||||||||||||||||||||||||||||||
| PSUs
| 2/12/2016
|
| 12,492
|
|
| 236,723
|
| 100% in 2019, subject to performance
| ||||||||||||||||||||||||||||||
| 2/14/2017
|
| 19,040
|
|
| 360,808
|
| 100% in 2020, subject to performance
| |||||||||||||||||||||||||||||||
Erez Vigodman
| Options
| 1/8/2014
|
| 187,134
|
|
| 93,568
|
|
| 41.05
|
|
| 1/7/2024
|
| 33% in 2016, 2017 and 2018
| |||||||||||||||||||||||
| 2/12/2015
|
| 54,619
|
|
| 54,619
|
|
| 57.35
|
|
| 2/11/2025
|
| 33% in 2017 and 2018 (2019 tranche forfeited)
| |||||||||||||||||||||||||
| 2/12/2016
|
| 58,276
|
|
| 55.75
|
|
| 2/11/2026
|
| 33% in 2018 (2019 and 2020 tranches forfeited)
| ||||||||||||||||||||||||||||
| 5/16/2016
|
| 18,540
|
|
| 50.43
|
|
| 5/15/2026
|
| 33% in 2018 (2019 and 2020 tranches forfeited)
| ||||||||||||||||||||||||||||
| RSUs
| 1/8/2014
|
| 5,220
|
|
| 98,919
|
| 33% in 2016, 2017 and 2018
| ||||||||||||||||||||||||||||||
Dr. Yitzhak Peterburg
| Options
| 8/31/2010
|
| 198,752
|
|
| 50.62
|
|
| 8/30/2020
|
| vested
| ||||||||||||||||||||||||||
| 2/14/2017
|
| 255,104
|
|
| 34.90
|
|
| 2/13/2027
|
| 33% in 2019, 2020 and 2021
| ||||||||||||||||||||||||||||
| RSUs
| 2/14/2017
|
| 48,185
|
|
| 913,106
|
| 33% in 2019, 2020 and 2021
| ||||||||||||||||||||||||||||||
| PSUs
| 2/14/2017
|
| 53,552
|
|
| 1,014,810
|
| 100% in 2020, subject to performance
| ||||||||||||||||||||||||||||||
Eyal Desheh
| Options
| 11/7/2011
|
| 198,003
|
|
| 41.72
|
|
| 11/6/2021
|
| vested
| ||||||||||||||||||||||||||
| 3/12/2014
|
| 65,720
|
|
| 32,861
|
|
| 48.76
|
|
| 3/11/2024
|
| 33% in 2016, 2017 and 2018
| |||||||||||||||||||||||||
| 2/12/2015
|
| 29,792
|
|
| 59,584
|
|
| 57.35
|
|
| 2/11/2025
|
| 33% in 2017, 2018 and 2019
| |||||||||||||||||||||||||
| 2/12/2016
|
| 99,904
|
|
| 55.75
|
|
| 2/11/2026
|
| 33% in 2018, 2019 and 2020
| ||||||||||||||||||||||||||||
| 5/16/2016
|
| 13,908
|
|
| 50.43
|
|
| 5/15/2026
|
| 33% in 2018, 2019 and 2020
| ||||||||||||||||||||||||||||
| 2/14/2017
|
| 113,382
|
|
| 34.90
|
|
| 2/13/2027
|
| 33% in 2019, 2020 and 2021
| ||||||||||||||||||||||||||||
| RSUs
| 2/14/2017
|
| 21,415
|
|
| 405,814
|
| 33% in 2019, 2020 and 2021
| ||||||||||||||||||||||||||||||
| PSUs
| 2/12/2016
|
| 19,219
|
|
| 364,200
|
| 100% in 2019, subject to performance
| ||||||||||||||||||||||||||||||
| 5/16/2016
|
| 2,672
|
|
| 50,634
|
| 100% in 2019, subject to performance
| |||||||||||||||||||||||||||||||
| 2/14/2017
|
| 23,801
|
|
| 451,029
|
| 100% in 2020, subject to performance
| |||||||||||||||||||||||||||||||
Dr. Rob Koremans
| Options
| 3/1/2012
|
| 250,001
|
|
| 45.29
|
|
| 2/28/2022
|
| vested
| ||||||||||||||||||||||||||
| 3/12/2014
|
| 65,720
|
|
| 32,861
|
|
| 48.76
|
|
| 3/11/2024
|
| 33% in 2016, 2017 and 2018
| |||||||||||||||||||||||||
| 2/12/2015
|
| 31,447
|
|
| 62,896
|
|
| 57.35
|
|
| 2/11/2025
|
| 33% in 2017, 2018 and 2019
| |||||||||||||||||||||||||
| 2/12/2016
|
| 99,904
|
|
| 55.75
|
|
| 2/11/2026
|
| 33% in 2018, 2019 and 2020
| ||||||||||||||||||||||||||||
| 5/16/2016
|
| 8,346
|
|
| 50.43
|
|
| 5/15/2026
|
| 33% in 2018, 2019 and 2020
| ||||||||||||||||||||||||||||
| 2/14/2017
|
| 141,728
|
|
| 34.90
|
|
| 2/13/2027
|
| 33% in 2019, 2020 and 2021
| ||||||||||||||||||||||||||||
| RSUs
| 2/14/2017
|
| 26,769
|
|
| 507,273
|
| 33% in 2019, 2020 and 2021
| ||||||||||||||||||||||||||||||
| PSUs
| 2/12/2016
|
| 19,219
|
|
| 364,200
|
| 100% in 2019, subject to performance
| ||||||||||||||||||||||||||||||
| 5/16/2016
|
| 1,603
|
|
| 30,377
|
| 100% in 2019, subject to performance
| |||||||||||||||||||||||||||||||
| 2/14/2017
|
| 29,751
|
|
| 563,781
|
| 100% in 2020, subject to performance
| |||||||||||||||||||||||||||||||
72 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Executive Compensation
Option Awards
|
Stock Awards
|
|
| Option Awards | Stock Awards |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Name
| Award
| Grant
| Number of
| Number of (2)
| Option
| Option
| Number
| Market
| Equity
| Equity ($) (6)
| Vesting Schedule (7)
| Award Type | Grant Date | Number of Securities Underlying Unexercised Options (#) Exercisable (1) | Number of Securities Underlying Unexercised Options (#) Unexercisable (2) | Option Exercise Price ($) | Option Expiration Date | Number of Shares or Units of Shares That Have Not Vested (#) (3) | Market Value of Shares or Units of Shares That Have Not Vested ($) (4) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) (5) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That have Not Vested ($) (6) | Vesting Schedule (7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Dr. Michael Hayden
| Options
| 5/9/2012
|
| 275,000
|
|
| 42.19
|
|
| 5/8/2022
|
| vested
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Eric Drapé | Options | 12/9/2013 | 25,005 |
| 40.15 | 12/9/2023 |
|
|
|
| vested | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3/12/2014
|
| 65,720
|
|
| 32,861
|
|
| 48.76
|
|
| 3/11/2024
|
| 33% in 2016, 2017 and 2018
|
| 3/12/2014 | 15,002 |
| 48.76 | 3/12/2024 |
|
|
|
| vested | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2/12/2015
|
| 31,447
|
|
| 62,896
|
|
| 57.35
|
|
| 2/11/2025
|
| 33% in 2017, 2018 and 2019
|
| 2/12/2015 | 54,623 |
| 57.35 | 2/12/2025 |
|
|
|
| vested | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2/12/2016
|
| 99,904
|
|
| 55.75
|
|
| 2/11/2026
|
| 33% in 2018, 2019 and 2020
|
| 2/12/2016 | 54,950 |
| 55.75 | 2/12/2026 |
|
|
|
| vested | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5/16/2016
|
| 16,687
|
|
| 50.43
|
|
| 5/15/2026
|
| 33% in 2018, 2019 and 2020
|
| 2/14/2017 | 41,576 | 20,788 | 34.90 | 2/14/2027 |
|
|
|
| 33% in 2019, 2020 and 2021 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2/14/2017
|
| 113,382
|
|
| 34.90
|
|
| 2/13/2027
|
| 33% in 2019, 2020 and 2021
|
| 9/18/2017 | 33,339 |
| 16.99 | 9/18/2027 |
|
|
|
| vested | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| RSUs
| 2/14/2017
|
| 21,415
|
|
| 405,814
|
| 33% in 2019, 2020 and 2021
|
| 2/9/2018 | 16,743 | 33,488 | 18.61 | 2/9/2028 |
|
|
|
| 33% in 2020, 2021 and 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSUs
| 2/12/2016
|
| 19,219
|
|
| 364,200
|
| 100% in 2019, subject to performance
| RSUs | 2/14/2017 |
|
|
|
| 3,926 | 37,886 |
|
| 33% in 2019, 2020 and 2021 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5/16/2016
|
| 3,207
|
|
| 60,773
|
| 100% in 2019, subject to performance
|
| 2/9/2018 |
|
|
|
| 13,135 | 126,753 |
|
| 33% in 2020, 2021 and 2022 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2/14/2017
|
| 23,801
|
|
| 451,029
|
| 100% in 2020, subject to performance
|
| 3/4/2019 |
|
|
|
| 35,820 | 345,663 |
|
| 33% in 2021, 2022 and 2023 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| 2/28/2020 |
|
|
|
| 78,057 | 753,250 |
|
| 25% in 2021, 2022, 2023 and 2024 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PSUs | 2/9/2018 |
|
|
|
| 27,643 | 266,755 |
|
| 100% in 2021 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| 5/11/2018 |
|
|
|
| 37,884 | 365,581 |
|
| 100% in 2021 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| 3/4/2019 |
|
|
|
|
|
| 39,787 | 383,945 | 100% in 2022, subject to performance | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
| 2/28/2020 |
|
|
|
|
|
| 21,683 | 209,241 | 100% in 2023, subject to performance | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (1) | Amounts disclosed in this column reflect the number of options granted to our NEOs that were subject to |
| (2) | Amounts disclosed in this column reflect the number of options granted to our NEOs that were subject to |
| (3) | Amounts disclosed in this column reflect the number of unvested RSUs granted |
| (4) | Amounts disclosed in this column reflect the market value of the RSUs and PSUs reported in the preceding column using the closing price of a Teva share as reported on the |
| (5) | Amounts disclosed in this column reflect the number of unearned and unvested PSUs held by our NEOs, based on achievement of all applicable performance goals at target level for the open performance cycle ending in 2021 and at threshold level for the open performance cycles ending in |
| (6) | Amounts disclosed in this column reflect the market value of the unvested PSUs held by our NEOs and reported in the preceding column using the closing price of a Teva share as reported on the |
| (7) | This column discloses the vesting dates of outstanding awards held by our NEOs at year |
| 70 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Executive Compensation
20172020 Option Exercises and Stock Vested
The table below shows the number of shares each of our NEOs acquired and the values they realized upon the vesting of PSUs and RSUs, during 2017.2020. Values are shown before payment of any applicable withholding taxes or brokerage commissions. There were no sharestock options exercised by the NEOs in 2017.2020.
Stock Awards | ||||||||
Name
| Number of
| Value ($) (2)
| ||||||
Michael McClellan |
|
1,355 |
|
|
30,827 |
| ||
Dr. Carlo de Notaristefani |
|
22,642 |
|
|
753,979 |
| ||
Dr. Hafrun Fridriksdottir |
|
10,252 |
|
|
270,496 |
| ||
Mark Sabag |
|
23,703 |
|
|
644,405 |
| ||
Erez Vigodman |
|
5,220 |
|
|
177,480 |
| ||
Dr. Yitzhak Peterburg |
|
14,369 |
|
|
237,376 |
| ||
Eyal Desheh |
|
22,642 |
|
|
753,979 |
| ||
Dr. Rob Koremans |
|
22,642 |
|
|
753,979 |
| ||
Dr. Michael Hayden |
|
22,642 |
|
|
753,979 |
| ||
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 73
Executive Compensation
|
| Stock Awards | |||||||
Name | Number of on Vesting (#) (1) | Value Vesting ($) (2) | ||||||
Kåre Schultz | 419,181 | 3,801,156 | ||||||
Dr. Hafrun Fridriksdottir | 40,337 | 490,065 | ||||||
Brendan O’Grady | 18,465 | 220,807 | ||||||
Eric Drapé | 23,583 | 289,038 | ||||||
| (1) | Amounts disclosed in this column reflect the number of PSUs and RSUs that vested during |
| (2) | Amounts disclosed in this column reflect the value realized upon vesting of the PSUs and RSUs, as calculated based on the price of a Teva share on the vesting date, multiplied by the number of shares underlying each award. |
20172020 Pension Benefits
None of our NEOs participate in or have accrued benefits under qualified ornon-qualified defined benefit plans sponsored by us.
20172020 Nonqualified Deferred Compensation
Name
| Plan Name
| Executive
| Company
| Aggregate
| Aggregate
| Aggregate
| ||||||||||||||||
Michael McClellan
| Supplemental Deferred Compensation Plan
|
| 121,476
|
|
| 16,933
|
|
| 26,269
|
|
| 0
|
|
| 228,560
|
| ||||||
Dr. Carlo de Notaristefani | Supplemental Deferred Compensation Plan
|
| 1,059,731
|
|
| 0
|
|
| 162,992
|
|
| 0
|
|
| 1,666,236
|
| ||||||
Defined Contribution Supplemental Executive Retirement Plan
|
| 0
|
|
| 125,460
|
|
| 80,664
|
|
| 0
|
|
| 582,120
|
| |||||||
Hafrun Fridriksdottir | Supplemental Deferred Compensation Plan
|
| 59,289
|
|
| 24,923
|
|
| 10,087
|
|
| 0
|
|
| 129,381
|
| ||||||
Name | Plan Name | Executive Contributions in Last FY ($) (1) | Company Contributions in Last FY ($) (2) | Aggregate Earnings in Last FY ($) (3) | Aggregate Withdrawals / Distributions ($) | Aggregate Balance at Last FY ($) (4) | ||||||||||||||||
Hafrun Fridriksdottir | Supplemental Deferred Compensation Plan |
| 127,167
|
|
| 113,609
|
|
| 118,186
|
|
| 0
|
|
| 888,427
|
| ||||||
| Actavis Executive Deferred Compensation Plan | 0 | 0 | 6,391 | 0 | 140,930 | |||||||||||||||||
Brendan O’Grady | Supplemental Deferred Compensation Plan | 0 | 0 | 12,588 | (11,407 | ) | 206,001 | |||||||||||||||
| Teva Neuroscience Deferred Compensation Plan | 0 | 0 | 27,382 | 0 | 315,084 | |||||||||||||||||
| (1) | Amounts disclosed in this column reflect elective deferrals made by our NEOs and are included in the amounts reported as “Salary” and “Non-Equity Incentive Plan Compensation,” as relevant, in the Summary Compensation Table |
| (2) | Amounts disclosed in this column are included within the amount reported in the “All Other Compensation” column of the Summary Compensation |
| (3) | Amounts disclosed in this column include earnings on the |
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 71 | |
Executive Compensation
| Summary Compensation Table because the Company does not credit above-market or preferential earnings on deferred compensation. |
| (4) | Amounts disclosed in this column reflect the cumulative value of the applicable NEO’s contributions and Company matching contributions, which have been included in the amounts reported as “Salary,” “Non-Equity Incentive Plan Compensation,” and “All Other Compensation,” as appropriate, in the applicable Summary Compensation Tables, and investment earnings thereon. |
74 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Executive Compensation
Teva’s North American subsidiaries provide a tax qualified defined contribution 401(k) Retirement Savings Plan for the benefit of employees. Under this plan, contribution amounts have been determined based on specified percentages of pay. The Internal Revenue Code limits the benefits that may be contributed into the 401(k) plan. As a complement to this plan, the Company maintains twoa supplemental retirement plansplan, the Supplemental Deferred Compensation Plan, to bridge the gap between legally mandated limits on qualified plan benefits and the retirement benefits offered at comparable public companies, and to provide participants with supplemental benefits. The two plans include the Supplemental Deferred Compensation Plan, which is a broad-based plan, and the Defined Contribution Supplemental Executive Retirement Plan (“DC SERP”), which is available to grandfathered U.S. executive officers (no new U.S. executive officers are enrolled in this plan). While the Company has formally funded the 401(k) plan match contribution, the Supplemental Deferred Compensation Plan and the DC SERP areis not formally funded.
Supplemental Deferred Compensation Plan
The Supplemental Deferred Compensation Plan is a nonqualified, unfunded deferred compensation plan under which certain eligible employees may defer up to 75% of base salary, annual bonuses and sales bonuses. The Company matches 100% of the first 6% of all eligible compensation deferred above the IRS qualified compensation limit, and makes restorative matching contributions to restore the Company match that were lost to the participant under the Retirement Savings Plan. Participants are vested in 100% of Company contributions once three years of service are completed. There are 27 investment options within the Supplemental Deferred Compensation Plan, and participants may change their investment allocations. Contributions plus earnings are paid out of the general assets of the Company. Participants that are age 55 with at least 15 years of service or age 65 with five years of service are retirement eligible, and may receive payment from the Plan in a lump sum or in annual installments for up to 20 years beginning on the first distribution date (January or July) that is at least 13 months after their retirement. Participants that terminate employment prior to retirement receive a lump sum beginning on the first distribution date that is at least six months after termination. Participants may change their distribution election at least 12 months prior to the originally scheduled payment date and as long as the change results in the payment date being delayed at least five years.
Defined Contribution SupplementalActavis Executive RetirementDeferred Compensation Plan
In connection with Teva’s acquisition of Actavis Generics in November 2016, Teva assumed the Actavis Executive Deferred Compensation Plan. Certain former Actavis employees remain participants in the plan, although the plan has been frozen and further participant deferrals into the plan are no longer permitted. The DC SERPplan is a nonqualified, unfunded deferred compensation plan inunder which certain executive officers may participate. Under thiseligible employees of Actavis Generics prior to its acquisition by Teva were able to defer up to 80% of base salary and 80% of performance bonus awards (100% after January 1, 2015). The plan thealso provided for Company establishes an account on behalf of each participantmatching credits, Company discretionary credits, and credits that account on the last day of the year with an amount equal to 15% of the participant’sand debits for investment returns. Participants are fully vested in their base salary paid during the year as a future retirement benefit. If the participant has a separation from service after age 65 or dies or becomes disabled, the Company will credit the account with apro-rata amount in respect of the portion of the year during which the participant qualified as a participant. The participant may direct percentages of the amounts credited to the participant’s account to be notionally invested in notional investment funds, and the account is credited with earnings that mirror the investment results of such investment funds. As of a valuation date, the notional realizedperformance bonus deferrals, and unrealized gains and losses and the notional income are allocated for the benefit of the participant’s account. Participants vest in their accountsCompany matching contributions after certain numbers of years. Participants become 100% vested upon either the earliest of five full years as a participant, attaining age 65 while employed by the Company, death or disability, orand upon a change in control as defined under Code Section 409A. Ifwill receive a participant separates from service before they are 100% vested, they will forfeit the entire account balance. If a participant breaches any noncompete or nonsolicit or other covenants under the plan, is terminated for cause or failssingle lump sum payment within 12 months. Participants may elect to execute a release of claims against the Company upon a termination of employment, they will forfeit their account balance. A participant may receive the vested benefit in the accountdistributions in a lump sum following their separationor in annual installments of from service or, iftwo to 15 years.
Teva Neuroscience Deferred Compensation Plan
The Teva Neuroscience Deferred Compensation Plan is a nonqualifed, unfunded deferred compensation plan for certain employees of the participant so elects, in installments. If a participant does not have 10 years of service and is 55 atCompany. Under the time of separation from service, payment will be inplan, during the formperiod 2001 to 2005, the Company contributed 10% of a single lump sum. If aparticipant’s total compensation up to the limits of Code Section 401 to an account for such participant. The participant dies after separation from service and priorwas neither permitted nor required to benefits being paid, such benefits will continuemake contributions to be paid in the same form as elected by the participant. If the participant dies or becomes disabled, the vested value of the account will be distributed in a single lump sum. If installment payments are elected,plan,
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 75
| 72 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Executive Compensation
and the installment amountsbalance in such participant’s account is fully vested at all times. There are determined as27 investment options within the remainingplan, and participants may change their investment allocations. Participants are entitled to a lump sum payment of their account balance divided byon the numberdate of years over which the installments will be paid. Payments may be delayed due to certain tax rulestheir retirement after reaching age 65, disability, death or deferral elections made by the executive.separation from service.
20172020 Potential Payments Upon Termination or Change in Control
In connection with any termination of employment, including if there is a termination in connection with a change in control of the Company, our NEO’sNEOs would be eligible to receive certain payments, benefits and treatment of the various forms of equity that suchthe NEO holds (provided, in some cases, that certain conditions are met).
The amounts that the NEOs would receive are set forth below for the following types of termination of employment: termination for cause, death, disability, retirement, termination without cause, resignation for good reason, resignation without good reason and a change in control of the Company.
In accordance with SEC rules, we have used certain assumptions in determining the amounts shown. We have assumed that the termination of employment or change in control occurred on December 31, 2017,2020, and that the value of a Teva share on that day was $18.95,$9.65, the closing price on the NYSE on December 29, 2017,31, 2020, the last trading day of 2017.2020.
Under these SEC rules, the potential payments upon termination do not include certain distributions or benefits which are not enhanced by a qualifying termination of employment or change in control. These payments and benefits are referred to as “vested benefits” and include:
| ∎ | Amounts payable when employment terminates under programs generally applicable to the Company’s salaried employees; |
| ∎ | Vested benefits accrued under the 401(k) and pension plans; and |
| ∎ | Vested benefits under the Supplemental Deferred Compensation Plan, Teva Neuroscience Deferred Compensation Plan and the |
Current NEOs
Kåre Schultz
Mr. Schultz’s employment terms generally require the Company and Mr. Schultz to provide three months’ notice of termination of employment, other than in connection with a non-renewal, which provides for one year notice, and in connection with termination for cause, death or disability. We may waive Mr. Schultz’s services during suchthe notice of termination period or any part thereof, or accelerate the termination date upon mutual agreement, on the condition that we pay him his monthly base salary and all additional compensation and benefits in respect of such waived period.
Mr. Schultz’s employment terms provide that in connection with his termination of employment, Mr. Schultz will be entitled to receive payments associated with termination as required pursuant to applicable Israeli law and certain accrued obligations. Upon termination by the Company without cause or by Mr. Schultz with good reason, Mr. Schultz will generally be entitled to receive cash severance, together with severance amounts accumulated in his severance account, equal to the product of twelve times his monthly base salary (or the minimum amount required under applicable law, if greater). Mr. Schultz is also entitled to receive an amount equal to twenty-four times his monthly base salary, in consideration for, and conditioned upon, his undertaking not to compete with Teva for two years following termination and other restrictive covenants, and his compliance with such undertaking, which amount would be paid in connection with terminations other than in the event of his termination by the Company for cause, non-renewalor his death. In the event that his employment is terminated by the Company without cause or by Mr. Schultz with good reason within one year following certain mergers and as a result thereof, Mr. Schultz will be entitled to an additional lump sum cash payment equal to his current annual salary.
76 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 73 | |
Executive Compensation
Upon his termination by the Company without cause, resignation by Mr. Schultz with good reason, non-renewal by the Company, and non-renewal by Mr. Schultz due to death, disability,his retirement, Mr. Schultz will receive continued vesting of outstanding awards in accordance with their terms. Upon his termination by the Company without cause and resignation by Mr. Schultz with good reason, Mr. Schultz will receive payment of any unvested portion of hissign-on cash award,accelerated vesting of hissign-on RSU award on the later of the date of termination. Upon his termination due to death and the first anniversary of the grant date, anddisability, default plan treatment will apply as described below except that Mr. Schultz will receive continued vesting of hissign-on PSU awardsaward (which will ultimately be settled based on actual performance through the end of the applicable three-year and five-year performance periods)period). In the event of a change in control before the terminations listed above, Mr. Schultz’ssign-on PSU awardsaward will be treated as earned based on the price paid per share to shareholders (or if none, then based on the last per share trading price before the change in control). The awardsaward may then either continue as a time-vested awardsaward over the remainder of the required vesting period or, if not assumed, settled upon the change in control. If thesign-on PSU awards areaward is assumed and continuecontinues as a time-vested awards, theyaward, it will be immediately settled upon termination following the change in control due to death, disability, termination without cause, and resignation with good reason.reason, non-renewal by the Company and non-renewal by Mr. Schultz due to his retirement.
All termination payments and benefits in excess of those required to be paid pursuant to applicable law are subject to the execution of a release of claims, and shall immediately terminate without further obligation of Teva, in the event that he breaches his restrictive covenants. In addition, in the event of continuous and willful breach of his restrictive covenants, the Company shall be entitled to a repayment of such termination payments, including forfeiture of any post-termination equity vesting.
Michael McClellanEli Kalif
Mr. McClellan’sKalif’s employment terms generally require the Company and Mr. McClellanKalif to provide threesix months’ notice of termination of employment, other than in connection with a termination for cause, death or disability. We may waive Mr. McClellan’sKalif’s services during such notice period or any part thereof, or accelerate the termination date, on the condition that we pay him his monthly base salary and all additional compensation and benefits in respect of such waived period.
Mr. Kalif’s employment terms provide that in connection with his termination of employment, Mr. Kalif will be entitled to receive payments associated with termination as required pursuant to applicable Israeli law and certain accrued obligations. Upon termination by the Company without cause or by Mr. McClellan forKalif with good reason, Mr. McClellanKalif will generally be entitled to receive cash severance, together with severance amounts accumulated in his severance account, equal to twice his monthly base salary multiplied by the productnumber of sixyears of employment, up to a maximum payment of eighteen times his monthly base salary and payment of certain costs associated with continued medical insurance for eighteen months. Mr. McClellan is also entitled to receive an(or the minimum amount equal to twelve times his monthly base salary, in consideration for, and conditioned upon, his undertaking not to compete with Teva for one year following termination and other restrictive covenants.required under applicable law, if greater). In the event that his employment is terminated by the Company without cause within one year following certain mergers anda change in control event (as such term is defined in the Compensation Policy as a result thereof,in effect on the date hereof), Mr. McClellanKalif will be entitled to an additional lump sum cash payment ofequal to $1.5 million.
All termination payments and benefits in excess of those required to be paid pursuant to applicable law are subject to the execution of a release of claims, and shall immediately terminate without further obligation of Teva, and Mr. McClellanKalif shall promptly repay Teva any such payments or benefits provided, in the event that he breaches his restrictive covenants.
Dr. Carlo de Notaristefani
Dr. de Notaristefani’s employment terms generally require the Company and Dr. de Notaristefani to provide six months’ notice of termination of employment, other than in connection with a termination for cause, death or disability. We may waive Dr. de Notaristefani’s services during such notice period or any part thereof, on the condition that we pay him his monthly base salary and all additional compensation and benefits in respect of such waived period.
Upon termination by the Company without cause, by Dr. de Notaristefani for good reason, or by Dr. de Notaristefani without good reason on or after July 1, 2020, Dr. de Notaristefani will generally be entitled to receive cash severance equal to the product of twelve times his monthly base salary and payment of certain costs associated with continued medical insurance for eighteen months. Dr. de Notaristefani is also entitled to receivecovenants, including an amount equal to twelve times his monthly base salary, in consideration for, and conditioned upon, his undertaking not to compete with Teva for one yearsix months following termination and other restrictive
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 77
Executive Compensation
covenants. In the event that his employment is terminated without cause within one year following certain mergers and as a result thereof, Dr. de Notaristefani will be entitled to an additional lump sum cash payment of $1.5 million.
Dr. de Notaristefani is also entitled to continued vesting in full of equity-based awards following termination without cause and continued vesting in full of equity-based awards following resignation with or without good reason on or after July 1, 2020.
All termination payments and benefits in excess of those required to be paid pursuant to applicable law are subject to the execution of a release of claims, and shall immediately terminate without further obligation of Teva, and Dr. de Notaristefani shall promptly repay Teva any such payments or benefits provided, in the event that he breaches his restrictive covenants.termination.
Dr. Hafrun Fridriksdottir
Dr. Fridriksdottir’semploymentFridriksdottir’semployment terms generally require the Company and Dr. Fridriksdottir to provide six months’ notice of termination of employment, other than in connection with a termination for cause, death or disability. We may waive Dr. Fridriksdottir’s services during such notice period or any part thereof, or accelerate the termination date, on the condition that we pay her the monthly base salary and all additional compensation and benefits in respect of such waived period.
| 74 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Executive Compensation
Upon termination by the Company without cause or by Dr. Fridriksdottir for good reason, Dr. Fridriksdottir will generally be entitled to receive cash severance equal to the product of twelve times her monthly base salary if terminated before August 3, 2018, and cash severance equal to the product of six times her monthly base salary if terminated on or after August 3, 2018. In addition, Dr. Fridriksdottir will be entitled toand payment of certain costs associated with continued medical insurance for eighteen months. Dr. Fridriksdottir is also entitled to receive an amount equal to twelve times her monthly base salary, in consideration for, and conditioned upon, her undertaking not to compete with Teva for one year following termination and other restrictive covenants. In the event that her employment is terminated without cause within one year following certain mergers and as a result thereof, Dr. Fridriksdottir will be entitled to an additional lump sum cash payment of $1.5 million.
Because Dr. Fridriksdottir meets the requirements for a qualifying retirement and termination under the Company’s policy pursuant to its 2015 Long-termand 2020 Long-Term Equity-Based Incentive Plan, if she is terminated without cause and the current retirement policy is in effect, she will be entitled to continued vesting of her outstanding awards granted by the Company after the acquisition of Actavis Generics. In addition, if she is terminated without cause, (or resigns for good reason) before August 3, 2018,reason, or resigns without good reason, she will also be entitled to immediate vestingcontinued exercisability of unvestedvested options until the earlier of the applicable expiration date or two years after termination for equity awards originally granted to her by Allergan plc and converted into Company equity awards at the time she joined the Company following Teva’s acquisition of Actavis Generics, (“Rollover Awards”). If she resigns without good reason, she will be entitled to continued exercisability of vested options until the earlier of the applicable expiration date or two years after termination for Rollover Awards only due to the legacy Allergan qualifying retirement policy.
All termination payments and benefits in excess of those required to be paid pursuant to applicable law are subject to the execution of a release of claims, and shall immediately terminate without further obligation of Teva, and Dr. Fridriksdottir shall promptly repay Teva any such payments or benefits provided, in the event that she breaches her restrictive covenants.
Mark SabagBrendan O’Grady
Mr. Sabag’sO’Grady’s employment terms generally require the Company and Mr. SabagO’Grady to provide ninethree months’ notice of termination of employment, other than in connection with a termination for cause, death or disability. We may waive Mr. Sabag’sO’Grady’s services during such notice period or any part thereof, or accelerate the termination date, on the condition that we pay him his monthly base salary and all additional compensation and benefits in respect of such waived period.
78 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Executive Compensation
Upon termination by the Company without cause or by Mr. Sabag’s employment terms provide that in connection with his termination of employment,O’Grady for good reason, Mr. SabagO’Grady will generally be entitled to receive payments associated with termination as required pursuant to applicable Israeli law. In the event of retirement to pension at the statutory age, termination due to death or disability, termination without cause, or resignation for good reason, Mr. Sabag will be entitled to amake-up paymentcash severance equal to his monthly base salary multiplied by the numberproduct of his years of service, that together with severance amounts accumulated in his pension insurance fund account cannot exceed twice his monthly base salary multiplied by the number of his years of service. In the event of a resignation without good reason, themake-up payment will be equal to half his monthly base salary multiplied by the number of his years of service, that together with severance amounts accumulated in his pension insurance fund account cannot exceed 1.5six times his monthly base salary multiplied by the numberand payment of his years of service.certain costs associated with continued medical insurance for eighteen months. Mr. SabagO’Grady is also entitled to receive an amount equal to twelve times his monthly base salary, in consideration for, and conditioned upon, his undertaking not to compete with Teva for one year following termination. This amount would not be paid upon termination for cause or death.and other restrictive covenants. In the event that his employment is terminated without cause within one year following certain mergers and as a result thereof, Mr. SabagO’Grady will be entitled to an additional lump sum cash payment of $1.5 million.
All termination payments and benefits in excess of those required to be paid pursuant to applicable law are subject to the execution of a release of claims, and shall immediately terminate without further obligation of Teva, and Mr. Sabag is also entitled to continued vesting of equity-based awards for twenty-four months following termination without cause. In addition,O’Grady shall promptly repay Teva any such payments or benefits provided, in the event that he breaches his employment is terminated without cause within one year following certain mergers and as a result thereof, restrictive covenants.
Eric Drapé
Mr. Sabag will be entitled to accelerated vesting of unvested equity upon termination.
Thenon-compete payment is subject to compliance with thenon-compete covenant. In the event of a material breach, payment will cease and the Company will be entitled to reclaim amounts already paid.
Former NEOs
For all of the former NEOs, theDrapé’s employment terms generally require the partiesCompany and Mr. Drapé to provide six months’ notice of termination of employment, (ranging from 6 to 9 months), other than in connection with a termination for cause, death or disability. We may waive Mr. Drapé’s services during such notice period or any part thereof, or accelerate the termination date, on the condition that we pay the executive thehim his monthly base salary and all additional compensation and benefits in respect of such waived period. We did not waive the notice period for any former NEO. All of the former NEOs received notice during 2017, and with the exception of Mr. Vigodman, who completed the notice period during 2017, all of the former NEOs will complete their notice periods during 2018.
Erez Vigodman
Pursuant to Mr. Vigodman’s terms of employment, in connection with his termination of employment, Mr. Vigodman was entitled to receive nine months’ notice, payments associated with termination as required pursuant to Israeli law, certain previously accrued obligations, including payout of accrued vacation, and a payment that, together with severance amounts accumulated in his existing pension insurance funds, equals the product of twice his monthly base salary multiplied by the number of his years of service. Mr. Vigodman is also receiving an amount equal to eighteen times his monthly base salary in consideration for compliance with certainnon-competition covenants.
Under his employment agreement, Mr. Vigodman is also entitled to continued vesting of equity-based awards for twelve months following termination.
The severance, other than statutory severance, thenon-compete payment and the equity benefits, are subject to compliance withnon-compete and other restrictive covenants. In the event of a breach, payment and vesting cease and in the event of a material breach the Company will be entitled to reclaim amounts of the non-compete payment already paid.
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 79
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 75 | |
Executive Compensation
Dr. Yitzhak Peterburg
Pursuant to Dr. Peterburg’sMr. Drapé’s employment terms of employment,provide that in connection with his termination of employment, Dr. Peterburg isMr. Drapé will be entitled to receive nine months’ notice, payments associated with termination as required pursuant to applicable Israeli law and certain previously accrued obligations, including payout of accrued vacation, and a payment that,obligations. Upon termination by the Company without cause or by Mr. Drapé with good reason, Mr. Drapé will generally be entitled to receive cash severance, together with severance amounts accumulated in his existing pension insurance funds, equalsseverance account, equal to the product of twicefifteen times his monthly base salary multiplied by(or the number of his years of service as Interim President and CEO.
Under his employment agreement, Dr. Peterburg willminimum amount required under applicable law, if greater). Mr. Drapé is also be entitled to continued vesting in full of all equity-based awards granted to him as Interim President and CEO.
The severance, other than statutory severance, and equity benefits are subject to compliance withnon-compete and other restrictive covenants. In the event of a breach, payment and vesting cease and in the event of a material breach the Company will be entitled to reclaim any such benefits.
Eyal Desheh
Pursuant to Mr. Desheh’s terms of employment, in connection with his termination of employment, Mr. Desheh is entitled to receive nine months’ notice, payments associated with termination as required pursuantan amount equal to Israeli law, certain previously accrued obligations, including payout of accrued vacation, amake-up payment equal tothree times his monthly base salary, multiplied by the number of his years of service, that together with severance amounts accumulated in his pension insurance fund account cannot exceed twice his monthly base salary multiplied by the number of his years of service,consideration for, and eligibility to apro-rata annual cash incentive for the term active in position. Mr. Desheh is also receiving an amount equal to twelve times his monthly base salary, conditioned upon, his undertaking not to compete with Teva for six months following termination and other restrictive covenants, and his compliance with such undertaking, which amount would be paid in connection with terminations other than in the event of his termination by the Company for cause, his death or disability. In the event that his employment is terminated by the Company without cause within one year following termination.
a change in control event (as such term is defined in the Compensation Policy as in effect on the date hereof), Mr. Desheh is also entitled to continued vesting in full of equity-based awards due to our qualifying retirement and qualifying termination policy.
Thenon-compete payment is subject to compliance with thenon-compete covenant. In the event of a material breach, payment will cease and the CompanyDrapé will be entitled to reclaim amounts already paid.
Dr. Rob Koremans
Pursuant to Dr. Koremans’ terms of employment, in connection with his termination of employment, Dr. Koremans is entitled to receive six months’ notice, a severancean additional lump sum cash payment equal to 12 monthly salaries$1.5 million.
All termination payments and target annual cash incentive (for a totalbenefits in excess of 24 monthly salaries).
Dr. Koremans is also entitledthose required to continued vesting of equity-based awards until March 1, 2020.
Dr. Michael Hayden
Pursuant to Dr. Hayden’s terms of employment, in connection with his termination of employment, Dr. Hayden is entitled to receive nine months’ notice, payments associated with termination as requiredbe paid pursuant to Israeliapplicable law certain previously accrued obligations, including payout of accrued vacation, a payment equal to 12 monthly salaries, a payment that, together with severance amounts accumulated in his existing pension insurance funds, equals the product of twice his monthly base salary multiplied by the number of his years of service, a payment equal to the premium for continued health insurance coverage for eighteen months following the termination date, and certain relocation benefits in the event of a move back to Canada within one year following the termination date.
Dr. Hayden is also entitled to continued vesting of certain equity-based awards due to our qualifying retirement and qualifying termination policy.
80 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Executive Compensation
The severance amount, other than statutory severance, as well as the medical benefits, equity benefits and the relocation benefits are subject to the execution of a release of claims. These paymentsclaims, and benefits (other than the components required to be paid under applicable law) shall immediately terminate without further obligation of Teva, and the CompanyMr. Drapé shall have no further obligations to Dr. Hayden with respect thereto,repay Teva any such payments or benefits provided, in the event that he breaches any provisions related to confidentiality or his covenant not to compete.restrictive covenants.
Potential Payments Upon Termination or Change In Control
The following tables summarize the payments the current NEOs would receive upon termination and completion of the required notice period at December 31, 2017 and the payments the former NEOs are eligible to receive upon termination and completion of the required notice period, as applicable. As the former NEOs have already received notice of termination, amounts for other termination events such as death, disability or change in control are not included.2020. The U.S. dollar amounts in the tables below were converted from local currency, where needed, using the December monthly average exchange rate of 3.503.25 Israeli shekels per U.S. dollar and 0.840.82 euros per U.S. dollar, except accrued vacation for Mr. Vigodman since this payment was made in November 2017 (monthly average exchange rate of 3.52 shekels per U.S. dollar).
Current NEOsdollar.
Payments Resulting From Termination without Cause or Resignation with Good Reason
Category | Kåre Schultz | Michael McClellan | Dr. Carlo de Notaristefani | Dr. Hafrun Fridriksdottir | Mark Sabag | |||||||||||||||
Severance payments (1)
|
|
1,972,120
|
|
|
350,000
|
|
|
836,400
|
|
|
720,000
|
|
|
872,505
|
| |||||
Non-compete payments (2)
|
|
4,000,000
|
|
|
700,000
|
|
|
836,400
|
|
|
720,000
|
|
|
621,262
|
| |||||
Accrued vacation
|
|
6,364
|
|
|
0
|
|
|
0
|
|
|
0
|
|
|
245,304
|
| |||||
Health benefits continuation
|
|
0
|
|
|
36,937
|
|
|
27,123
|
|
|
11,254
|
|
|
0
|
| |||||
Sign-on cash award (3)
|
|
20,000,000
|
|
|
0
|
|
|
0
|
|
|
0
|
|
|
0
|
| |||||
Post termination equity vesting (4)(5)
|
|
33,173,510
|
|
|
0
|
|
|
2,093,558
|
|
|
1,190,723
|
|
|
344,928
|
| |||||
Total amount without merger
|
$
|
59,151,994
|
|
$
|
1,086,937
|
|
$
|
3,793,481
|
|
$
|
2,641,977
|
|
$
|
2,083,999
|
| |||||
Post-merger termination payment (6)
|
|
2,000,000
|
|
|
1,500,000
|
|
|
1,500,000
|
|
|
1,500,000
|
|
|
1,500,000
|
| |||||
Post-merger equity acceleration (7)
|
|
0
|
|
|
0
|
|
|
0
|
|
|
0
|
|
|
922,183
|
| |||||
Total amount with merger
|
$
|
61,151,994
|
|
$
|
2,586,937
|
|
$
|
5,293,481
|
|
$
|
4,141,977
|
|
$
|
4,506,182
|
| |||||
Category | Kåre Schultz | Eli Kalif | Dr. Hafrun Fridriksdottir | Brendan O’Grady | Eric Drapé | |||||||||||||||
Severance payments (1) | 1,425,832 | 59,920 | 360,000 | 400,000 | 890,787 | |||||||||||||||
Non-compete payments (2) | 4,000,000 | 0 | 720,000 | 800,000 | 188,692 | |||||||||||||||
Accrued vacation | 30,951 | 69,149 | 0 | 0 | 135,547 | |||||||||||||||
Health benefits continuation | 0 | 0 | 13,074 | 43,888 | 0 | |||||||||||||||
Post-termination equity vesting (3)(4) | 25,156,913 | 0 | 4,305,261 | 0 | 0 | |||||||||||||||
Total amount without change in control | $ | 30,613,696 | $ | 129,069 | $ | 5,398,335 | $ | 1,243,888 | $ | 1,215,026 | ||||||||||
Post-change in control cash termination payment (5) | 2,000,000 | 1,500,000 | 1,500,000 | 1,500,000 | 1,500,000 | |||||||||||||||
Total amount with change in control | $ | 32,613,696 | $ | 1,629,069 | $ | 6,898,335 | $ | 2,743,888 | $ | 2,715,026 | ||||||||||
| (1) | In addition to the amounts reported above, Mr. Schultz would receive |
| (2) | For Mr. Schultz and Mr. Drapé, thenon-compete payment would be paid, assuming |
| (3) |
Amounts reported are based on the price of a Teva share on December |
| (4) | For Mr. Schultz, the equity vesting also applies in the event of non-renewal by the Company or non-renewal by Mr. Schultz due to retirement. For Dr. Fridriksdottir, the equity vesting does not apply to resignation with |
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 81
| 76 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Executive Compensation
| (5) | For Mr. Schultz, |
| or by Mr. |
Accelerated/ContinuedAccelerated Equity Vesting Upon Death or Disability
Under our 2015 and 2020 Long-Term Equity-Based Incentive Plan, upon death or disability, performance awards, such as PSUs, will immediately vest and pay out based on the target level of performance as of the date of termination, RSUs will immediately be vested and settled and options will immediately vest and remain exercisable through the original expiration date. For treatment of Mr. Schultz’ssign-on equity awards upon death or disability, see the summary of his termination terms above.
Under our 2010 Long-Term Equity-Based Incentive Plan, upon death or disability, RSUs, restricted shares and options will continue to vest, as if no termination had occurred, and will remain exercisable through their original expiration date or settle in accordance with the schedule set forth in the applicable award agreement.
Category | Kåre Schultz | Michael McClellan | Dr. Carlo de Notaristefani | Dr. Hafrun Fridriksdottir | Mark Sabag | Kåre Schultz | Eli Kalif | Dr. Hafrun Fridriksdottir | Brendan O’Grady | Eric Drapé | ||||||||||||||||||||||||||||||
Value (1) | $ | 45,280,738 | $ | 245,126 | $ | 2,093,558 | $ | 1,190,723 | $ | 922,183 | $ | 25,156,913 | $ | 1,693,758 | $ | 4,305,261 | $ | 4,164,293 | $ | 3,116,776 | ||||||||||||||||||||
| (1) | Amounts reported are based on the price of a Teva share on December |
Former NEOs
Payments Resulting From Termination without Cause
Category | Erez Vigodman | Dr. Yitzhak Peterburg | Eyal Desheh | Dr. Rob Koremans | Dr. Michael Hayden | |||||||||||||||
Severance payments (1)
|
|
528,836
|
|
|
286,549
|
|
|
967,056
|
|
|
1,641,994
|
|
|
1,658,877
|
| |||||
Non-compete payments (1)
|
|
2,511,139
|
|
|
0
|
|
|
854,289
|
|
|
0
|
|
|
0
|
| |||||
Accrued vacation (1)
|
|
370,195
|
|
|
174,396
|
|
|
113,416
|
|
|
0
|
|
|
335,523
|
| |||||
Health benefits continuation
|
|
0
|
|
|
0
|
|
|
0
|
|
|
0
|
|
|
18,666
|
| |||||
Post termination equity vesting (2)
|
|
98,919
|
|
|
1,927,916
|
|
|
1,271,678
|
|
|
1,296,540
|
|
|
560,238
|
| |||||
Total
|
$
|
3,509,089
|
|
$
|
2,388,861
|
|
$
|
3,206,439
|
|
$
|
2,938,534
|
|
$
|
2,573,304
|
| |||||
82 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 77 | |
HR and Compensation Committee Interlocks and Insider Participation
The HR and Compensation Committee currently consists of Rosemary A. Crane (chair), Jean-Michel Halfon, Gerald M. Lieberman, and Nechemia (Chemi) J. Peres.Peres and Janet S. Vergis. During fiscal year 2017,2020, no member of the HR and Compensation Committee was an employee, officer or former officer of Teva or any of its subsidiaries. During fiscal year 2017,2020, no member of the HR and Compensation Committee had a relationship that must be described under the SEC rules relating to disclosure of related party transactions. During fiscal year 2017,2020, none of our executive officers served on the Board of Directors or compensation committee of any entity that had one or more of its executive officers serving on Teva’s Board of Directors or HR and Compensation Committee.
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 83
| 78 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Proposal 2: Advisory Vote on Compensation of Named Executive Officers
As required by the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 (the “Dodd-Frank Act”) and Schedule 14A of the U.S. Securities Exchange Act of 1934, as amended (the “Exchange Act”), we are providing our shareholders with the opportunity to approve, by advisory vote, the compensation of our named executive officers,NEOs, as disclosed in this proxy statementProxy Statement in accordance with the rules of the SEC.
This proposal, commonly referred to as the“say-on-pay” vote, gives our shareholders the opportunity to express their views on the compensation of our named executive officers.NEOs. This vote is not intended to address any specific item of compensation or any specific named executive officer,NEOs, but rather the overall compensation of our named executive officersNEOs and our executive compensation philosophy, objectives and program, as described in this proxy statement.Proxy Statement. Accordingly, we ask our shareholders to approve the compensation of our named executive officers,NEOs, as disclosed pursuant to Item 402 of RegulationS-K of the Exchange Act in the section entitled “Executive Compensation” of this proxy statement,Proxy Statement, including the Compensation Discussion and Analysis,CD&A, the compensation tables and the related narrative disclosure, by casting anon-binding advisory vote “FOR” the following resolution:
“RESOLVED, that the shareholders of Teva Pharmaceutical Industries Limited approve, on anon-binding advisory basis, the compensation of its named executive officers, as disclosed pursuant to Item 402 of RegulationS-K, including the Compensation Discussion and Analysis, compensation tables and narrative discussion.”
As an advisory vote, the result will not be binding on the Board or the HR and Compensation Committee. Thesay-on-pay vote will, however, provide us with important feedback from our shareholders about our executive compensation philosophy, objectives and program. Our Board and the HR and Compensation Committee value the opinions of our shareholders and expect to take into account the outcome of the vote when considering future executive compensation decisions and when evaluating our executive compensation program. Following our 2021 Annual Meeting, the next advisory vote on named executive officer compensation is expected to occur at the 2022 annual meeting, unless the Board of Directors modifies its policy on the frequency of holding such advisory votes.
 |
The Board of Directors recommends that shareholders vote FOR the approval, on anon-binding advisory basis, of the compensation of Teva’s named executive officers, as disclosed in this |
84 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 79 | |
Proposal 3: Advisory Vote on Frequency of Advisory Vote on Compensation of Named Executive Officers
The Dodd-Frank Act and Schedule 14A of the Exchange Act also enable our shareholders to indicate, at least once every six years, how frequently we should seek an advisory vote on named executive officer compensation, such as Proposal 2 above. By voting on this Proposal 3, shareholders may indicate whether they would prefer an advisory vote on named executive compensation once every one, two or three years.
After careful consideration, our Board has determined that an advisorysay-on-pay vote should be held annually. Our Board believes that holding asay-on-pay vote annually is the most appropriate option because it will give us more frequent feedback from our shareholders on our executive compensation philosophy, objectives and program, as well as the compensation paid to our named executive officers.
For the reasons discussed above, our Board recommends that you vote for every “1 Year” as the frequency of futuresay-on-pay votes.
You may cast your advisory vote on the frequency of futuresay-on-pay votes by choosing the option of “1 Year,” “2 Years” or “3 Years,” or you may abstain from voting on this Proposal 3. The option of “1 Year,” “2 Years” or “3 Years” that receives the highest number of votes from shareholders present in person or represented by proxy and entitled to vote at the Annual Meeting will be deemed to be the frequency of futuresay-on-pay votes recommended by our shareholders. Abstentions will have no effect on this proposal.
While our Board believes that its recommendation is appropriate at this time, the shareholders are not voting to approve or disapprove the recommendation, but are instead asked to recommend, on anon-binding advisory basis, whether thesay-on-pay vote should be held once every one, two or three years.
Our Board and our Compensation Committee value the opinions of our shareholders on this matter and, to the extent there is any significant vote in favor of one time period over another, will take into account the outcome of this vote when making decisions regarding the frequency of holding futuresay-on-pay votes. However, because this vote is advisory and not binding on Teva, the Board or the Compensation Committee in any way, the Board may decide that it is in the best interests of our shareholders and Teva to hold asay-on-pay vote more or less frequently than the option recommended by our shareholders.
|
|
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 85
Proposal 4: Appointment of Independent Registered Public Accounting Firm
The Audit Committee recommends that, as required under Israeli law, shareholders appoint Kesselman & Kesselman, an independent registered public accounting firm in Israel and a member of PricewaterhouseCoopers International Limited (“PwC”), as Teva’s independent registered public accounting firm until the 2019 Annual MeetingTeva’s 2022 annual meeting of Shareholders.shareholders. PwC has been our independent registered public accounting firm since at least 1976, when Teva Pharmaceutical Industries Limited was established through the merger of several predecessor companies.1976.
Pursuant to Teva’s Articles of Association, the Board of Directors is authorized to fixdetermine the remuneration of Teva’s independent registered public accounting firm.
Representatives of PwC are expected to be present at the Annual Meeting and will also be available to respond to questions from shareholders. They also will have the opportunity to make a statement if they desire to do so.
Audit Committee Report
The Audit Committee has reviewed and discussed with management Teva’s audited consolidated financial statements as of and for the year ended December 31, 2017.2020.
The Audit Committee has also discussed with Kesselman & Kesselman the matters required to be discussed by the Public Company Accounting Oversight Board (PCAOB)(“PCAOB”) Auditing Standard No. 1301, Communications with Audit Committees.
The Audit Committee has received and reviewed the written disclosures and the letter from Kesselman & Kesselman required by the applicable requirements of the PCAOB regarding Kesselman & Kesselman’s communication with the Audit Committee concerning independence and has discussed with Kesselman & Kesselman their independence.
Based on the reviews and discussions referred to above, the Audit Committee recommended to the Board of Directors that the audited consolidated financial statements referred to above be included in Teva’s Annual Reporton Form 10-K for the year ended December 31, 20172020 for filing with the SEC.
Audit Committee of the Board of Directors
Gerald M. Lieberman, Chair
Amir Elstein
MurrayAbbas Hussain
Roberto A. Goldberg
Galia Maor
Dan S. SuesskindMignone
Policy onPre-Approval of Audit andNon-Audit Services of Independent Auditors
Teva’s Audit Committee is responsible for overseeing the work of its independent auditors. The Audit Committee’s policy is topre-approve all audit andnon-audit services provided by PwC and other members of PricewaterhouseCoopers International Limited. These services may include audit services, audit-related services, tax services and other services, as further described below. The Audit Committee sets forth the basis for itspre-approval in detail, listing the particular services or categories of services which arepre-approved, and setting forth a specific budget for such services. Tax services and otherOther services are approved by the Audit Committee on an individual basis. Once services have beenpre-approved, PwC and management then report to the Audit Committee on a periodic basis regarding the extent of services
86 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Proposal 4: Appointment of Independent Registered Public Accounting Firm
actually provided in accordance with the applicablepre-approval, and regarding the fees for the services performed. Such fees for 20172020 and 20162019 werepre-approved by the Audit Committee in accordance with these procedures.
| 80 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Proposal 3: Appointment of Independent Registered Public Accounting Firm
Principal Accountant Fees and Services
Teva paidincurred the following fees for professional services rendered by PwC and other members of PricewaterhouseCoopers International Limited, for the years ended December 31, 20172020 and 2016:2019:
| 2017 | 2016 | 2020 | 2019 | |||||||||||||
| (U.S. $ in thousands) | (U.S. $ in thousands) | |||||||||||||||
Audit fees | $ | 16,500 | $ | 18,495 | $ | 15,639 | $ | 17,280 | ||||||||
Audit-related fees | 482 | 505 | 571 | 480 | ||||||||||||
Tax fees | 4,025 | 8,490 | 3,820 | 3,445 | ||||||||||||
All other fees | 325 | 623 | 4 | 266 | ||||||||||||
Total | $ | 21,332 | $ | 28,113 | $ | 20,034 | $ | 21,472 | ||||||||
The audit fees for the years ended December 31, 20172020 and 20162019 were for professional services rendered for the integrated audit of Teva’s annual consolidated financial statements and its internal control over financial reporting as of December 31, 20172020 and 2016,2019, review of consolidated quarterly financial statements, statutory audits of Teva and its subsidiaries, issuance of comfort letters, consents and assistance with review of documents filed with the SEC, as well as the audit of carve out financial statements prepared in connection with certain divestment activities.SEC.
The Audit-relatedaudit-related fees for the years ended December 31, 20172020 and 20162019 were paid for the following services: sale side due diligence related to mergers and acquisitions,dispositions, accounting consultations and employee benefit plan audits, internal control reviews, attest services that are not required by statute or regulation and consultations concerning financial accounting and reporting standards.
Tax fees for the years ended December 31, 20172020 and 20162019 were paid for the following services: (i) services related to tax compliance, including the preparation of tax returns and claims for refund, (ii) tax planning and tax advice, including assistance with tax audits and appeals, (iii) advice related to mergers and acquisitions, (iv) tax services for employee benefit plans, and(v) assistance with respect to requests for rulings from tax authorities.authorities and (vi) other permitted tax services requested by management.
All other fees for the years ended December 31, 20172020 and 20162019 were paid mainly for an internal control review associated with the design and implementation plans of an ERP system,providing general advice related to new processes, as well as for license fees for the use of accounting research tools and training regarding general financial reporting developments.
The Audit Committee believes that the provision of allnon-audit services rendered is compatible with maintaining PwC’s independence.

| The Board of Directors recommends that shareholders vote FOR the approval of the appointment of Kesselman & Kesselman, a member of PwC, as Teva’s independent registered public accounting firm until |
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 87
Proposal 5: Amendment and Restatement of Teva’s 2008 Employee Stock Purchase Plan for U.S. Employees
The Board of Directors is submitting for approval by our shareholders the Teva Pharmaceutical Industries Limited 2008 Employee Stock Purchase Plan for U.S. Employees, as amended and restated effective September 7, 2017 (the “ESPP”), which amends and restates the Teva Pharmaceutical Industries Limited 2008 Employee Stock Purchase Plan for U.S. Employees as amended and restated effective January 1, 2016, which was originally approved at our 2008 annual shareholder meeting. The ESPP is effective for periods commencing September 7, 2017. The ESPP was amended and restated effective September 7, 2017 in order to (i) extend the term of the ESPP for a term of ten years from the effective date of the September 2017 amendment, (ii) increase the maximum number of ADSs authorized for issuance under the ESPP by an additional 5,000,000 ADSs, and (iii) make certain other ministerial changes to the ESPP.
The purpose of the ESPP is to provide employees of United States subsidiaries of Teva with the opportunity to purchase our ADSs, each representing one ordinary share, NIS 0.1 par value per share, through accumulated payroll deductions. The ESPP is intended to qualify under Section 423 of the Code. A summary of the material terms of the ESPP appears below. This summary is qualified in its entirety by the text of the ESPP, which is included asAppendix A to this proxy statement.
Administration
The ESPP will be administered by the compensation committee of the board of directors of Teva Pharmaceuticals USA, Inc. (“Teva USA”). Subject to the provisions of the ESPP, the Teva USA compensation committee will have the discretionary authority to construe, interpret and adjudicate all disputed claims under the ESPP.
Shares Available Under ESPP
Up to an aggregate of 8,500,000 ADSs (representing 8,500,000 ordinary shares) may be purchased by participants under the ESPP, subject to adjustments upon changes in capitalization of Teva as provided in the ESPP. Such ADSs (as well as the underlying shares) may be either newly-issued or already outstanding. The closing price of the ADSs on the NYSE on April 23, 2018 was $17.85 per ADS.
Eligible Participants
Employees of each U.S. subsidiary of Teva are eligible to participate in the ESPP if they are employed by such a subsidiary on the first trading day of each offering period. However, no employee is eligible to participate who, after the grant of options under the ESPP, would own (including all ADSs which may be purchased under any outstanding options under the ESPP) five percent (5%) or more of the total combined voting power or value of all classes of stock of Teva. As of April 23, 2018, the number of employees eligible to participate in the ESPP was 6,917.
Participation
The ESPP provides for consecutive three-month offering periods (or other offering periods as determined by the board of directors of Teva USA) during which participating employees may authorizeafter-tax payroll deductions of a specific whole percentage of his or her compensation between 1% and 10%, subject to a limit of the amount that will purchase no more than $25,000 of ADSs in any one calendar year based on the fair market value of the ADSs measured as of the first trading day in the applicable offering period (the “enrollment date”).
Offering
On each enrollment date, each participating employee will be granted an option to purchase, on the last day of the applicable offering period (each, an “exercise date”), the number of ADSs that may be
88 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Proposal 5: Amendment and Restatement of Teva’s 2008 Employee Stock Purchase Plan for U.S. Employees
purchased with the accumulated payroll deductions in his or her account as of the applicable exercise date. The purchase price for each option will be 95% of the fair market value of the ADSs on the exercise date. Unless a participant withdraws from the ESPP, his or her accumulated payroll deductions will automatically be used to exercise the options on the applicable exercise date.
Upon a participant’s ceasing to be an employee of a United States subsidiary of Teva for any reason, he or she will be deemed to have elected to withdraw from the ESPP, and such participant’s account balance will be distributed to him or her as soon as practicable. No further ADSs will be purchased for such participant.
Adjustments Upon Changes in Capitalization
The number of ADSs that may be purchased pursuant to options granted under the ESPP and the price per share of each option under the ESPP that has not yet been exercised shall be proportionately adjusted by the Board of Directors for any increase or decrease in the number of issued ADSs resulting from a stock split, reverse stock split, stock dividend, combination or reclassification of the ADSs, or any other increase or decrease in the number of ADSs effected without receipt of consideration by Teva. In the event Teva proposes to merge with or into another corporation or proposes to sell all or substantially all of its assets, each option under the ESPP shall be assumed, or an equivalent option shall be substituted by the successor or amalgamated corporation, unless the Board of Directors elects to shorten the offering period then in effect by setting a new exercise date. The Board of Directors may also make provision for adjusting the number of ADSs reserved under the ESPP, as well as the price per ADS covered by each outstanding option, in the event Teva effects one or more reorganizations, recapitalizations, rights offerings or other increases or reductions of its outstanding ADSs or ordinary shares, and in the event of Teva being consolidated with or merged into any other corporation.
Amendment and Termination
The Board of Directors may at any time and for any reason terminate or amend the ESPP without shareholder approval, except as required to satisfy Section 423 of the Code. Except as provided above in “Adjustments Upon Changes in Capitalization,” no termination or amendment may adversely affect options previously granted; provided, that an offering period may be terminated by the Board of Directors on any exercise date if it determines that the termination of the ESPP is in the best interests of Teva and its shareholders.
The amended and restated ESPP became effective (subject to the approval by the shareholders) upon its adoption by the Board of Directors on September 7, 2017 and will continue in effect for a term of ten years thereafter unless all ADSs authorized to be sold under it have been exhausted or the plan is otherwise terminated sooner.
Federal Income Tax Consequences
Generally, no federal income tax consequences will arise at the time an employee purchases ADSs under the ESPP. If an employee disposes of ADSs purchased under the ESPP less than one year after the ADS is purchased or within two years of the offering date, the employee will be deemed to have received compensation taxable as ordinary income for the taxable year in which the disposition occurs in the amount of the difference between the fair market value of the ADS at the time of purchase and the amount paid by the employee for the ADS. The amount of such ordinary income recognized by the employee will be added to the employee’s basis in the ADS for purposes of determining capital gain or loss upon the disposition of the ADS by the employee.
If an employee does not dispose of the ADSs purchased under the ESPP until at least one year after the ADSs are purchased and at least two years after the offering date, the employee will be deemed to have received compensation taxable as ordinary income for the taxable year in which the disposition occurs in an
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 89
Proposal 5: Amendment and Restatement of Teva’s 2008 Employee Stock Purchase Plan for U.S. Employees
amount equal to the lesser of (a) the excess of the fair market value of the ADSs on the date of disposition over the purchase price paid by the employee, and (b) the excess of the fair market value of the ADSs on the offering date over the purchase price paid by the employee. The amount of such ordinary income recognized by the employee will be added to the employee’s basis in the ADSs for purposes of determining capital gain or loss upon the disposition of the ADSs by the employee. If an employee dies before disposing of the ADSs purchased under the ESPP, he or she will be deemed to have received compensation taxable as ordinary income in the taxable year closing with the employee’s death in an amount equal to the lesser of clauses (a) and (b) as set forth in the first sentence of this paragraph. The employee will not realize any capital gain or loss at death.
We generally will not be entitled to a deduction with respect to the ADSs purchased by an employee under the ESPP, unless the employee disposes of the ADSs less than one year after the ADSs are transferred to the employee or less than two years after the offering date.
New Plan Benefits
No currentnon-employee directors will receive any benefit under the ESPP. The benefits that will be received, or that would have been received during the fiscal year ended December 31, 2017 if the ESPP had been in effect during such fiscal year, under the ESPP by our current executive officers and by all eligible employees are not currently determinable because the benefits depend upon the degree of participation by employees and, with respect to future benefits, the trading price of the ADSs in future periods.
 | ||
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement |
| |
90 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Presentation of 20172020 Financial Statements
The Board of Directors has approved, and is presenting to shareholders for receipt and consideration at the Annual Meeting, Teva’s annual consolidated financial statements for the year ended December 31, 2017,2020, which are included in Teva’s annual reportAnnual Report on Form10-K for the year ended December 31, 2017,2020, available on Teva’s website at www.tevapharm.com.
Section 16(a) Beneficial Ownership Reporting Compliance
As of January 1, 2018, Section 16(a) of the Exchange Act requires our directors and executive officers and persons who own more than 10% of our ordinary shares to file with the SEC initial reports of beneficial ownership and reports of changes in beneficial ownership of our ordinary shares. During 2017, our directors and executive officers and persons who own more than 10% of our ordinary shares were not required to comply with the reporting requirements of Section 16(a) because Teva was exempt from these requirements by virtue of being a “foreign private issuer.”
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 91
| 82 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
The following table describes, as of April 12, 2018,1, 2021, the beneficial ownership of Teva ordinary shares (and ADSs representing ordinary shares) by:
| ∎ | each person we believe beneficially holds more than 5% of the outstanding ordinary shares based solely on our review of SEC filings; |
| ∎ | each of our |
| ∎ | each of our directors and director nominees; and |
| ∎ | all of our directors and executive officers as a group. |
Beneficial Owner | Ordinary Shares Beneficially Owned *** | Percent of Ordinary Shares Outstanding **** | ||||||||
Beneficial Owners of More than 5% of Our Ordinary Shares
| ||||||||||
Capital Research and Management Company (1)
|
|
162,196,740
|
|
|
15.93
|
%
| ||||
Franklin Resources, Inc. (2)
|
|
70,601,691
|
|
|
6.93
|
%
| ||||
Named Executive Officers and Directors:*
| ||||||||||
Dr. Sol J. Barer
|
|
—
|
|
|
*
|
*
| ||||
Kåre Schultz
|
|
—
|
|
|
*
|
*
| ||||
Rosemary A. Crane
|
|
5,850
|
|
|
*
|
*
| ||||
Amir Elstein
|
|
1,993,706
|
|
|
*
|
*
| ||||
Murray A. Goldberg
|
|
—
|
|
|
*
|
*
| ||||
Jean-Michel Halfon
|
|
—
|
|
|
*
|
*
| ||||
Gerald M. Lieberman
|
|
5,400
|
|
|
*
|
*
| ||||
Galia Maor
|
|
—
|
|
|
*
|
*
| ||||
Roberto A. Mignone
|
|
750,000
|
(3)
|
|
*
|
*
| ||||
Dr. Perry D. Nisen
|
|
—
|
|
|
*
|
*
| ||||
Nechemia (Chemi) J. Peres
|
|
—
|
|
|
*
|
*
| ||||
Prof. Ronit Satchi-Fainaro
|
|
—
|
|
|
*
|
*
| ||||
Dan S. Suesskind
|
|
318,836
|
|
|
*
|
*
| ||||
Gabrielle Sulzberger
|
|
—
|
|
|
*
|
*
| ||||
Michael McClellan
|
|
30,833
|
|
|
*
|
*
| ||||
Dr. Carlo de Notaristefani
|
|
346,980
|
|
|
*
|
*
| ||||
Dr. Hafrun Fridriksdottir
|
|
25,423
|
|
|
*
|
*
| ||||
Mark Sabag
|
|
297,992
|
|
|
*
|
*
| ||||
Eyal Desheh (4)
|
|
384,133
|
|
|
*
|
*
| ||||
Dr. Michael Hayden (4)
|
|
475,339
|
|
|
*
|
*
| ||||
Dr. Rob Koremans (4)
|
|
484,081
|
|
|
*
|
*
| ||||
Dr. Yitzhak Peterburg (4)
|
|
228,215
|
|
|
*
|
*
| ||||
Erez Vigodman (4)
|
|
482,813
|
|
|
*
|
*
| ||||
All directors and executive officers as a group (24 persons)
|
|
4,368,230
|
|
|
*
|
*
| ||||
92 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
Security Ownership
Beneficial Owner | Ordinary Shares Beneficially Owned*** | Percent of Ordinary Shares Outstanding**** | ||||||
Beneficial Owners of More than 5% of Our Ordinary Shares |
|
|
|
|
|
| ||
Capital Research Global Investors (1) | 130,140,247 | 11.8 | % | |||||
Named Executive Officers and Directors:* |
|
|
|
|
|
| ||
Dr. Sol J. Barer | 277,433 | * | * | |||||
Kåre Schultz | 1,657,783 | * | * | |||||
Rosemary A. Crane | 33,509 | * | * | |||||
Amir Elstein | 2,024,225 | * | * | |||||
Jean-Michel Halfon | 30,519 | * | * | |||||
Abbas Hussain | — | * | * | |||||
Gerald M. Lieberman | 35,919 | * | * | |||||
Roberto A. Mignone | 1,525,577 | (2) | * | * | ||||
Dr. Perry D. Nisen | 25,577 | * | * | |||||
Nechemia (Chemi) J. Peres | 25,577 | * | * | |||||
Prof. Ronit Satchi-Fainaro | 17,621 | * | * | |||||
Janet S. Vergis | — | * | * | |||||
Eli Kalif | 18,430 | * | * | |||||
Dr. Hafrun Fridriksdottir | 421,187 | * | * | |||||
Brendan O’Grady | 250,158 | * | * | |||||
Eric Drapé | 350,562 | * | * | |||||
All directors and executive officers as a group (20 persons) | 8,194,567 | * | * | |||||
| * | The address of each named executive officer and director is c/o Teva Pharmaceutical Industries Limited, |
| ** | Represents less than 1%. |
| *** | For purposes of this table, “beneficial ownership” is determined in accordance with Rule13d-3 under the Exchange Act pursuant to which a person or group of persons is deemed to have “beneficial ownership” of any ordinary shares with respect to which such person has (or has the right to acquire within 60 days) sole or shared voting power or investment power. |
| **** | Percentage of beneficial ownership is based on |
| (1) | Based solely on a |
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 83 | |
Security Ownership
| the beneficial owner of 130,140,247 ordinary shares. Capital Research |
| (2) | 1,500,000 ordinary shares are |
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 93
| 84 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Securities Authorized for Issuance Underunder Equity Compensation Plans
The following table sets forth, as of December 31, 2017,2020, certain information related to our equity compensation plans:
Plan Category | Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights (a) | Weighted- Average Exercise Price of Outstanding Options, Warrants and Rights (b) | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a)) | Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights (a) | Weighted- Average Exercise Price of Outstanding Options, Warrants and Rights (b) | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a)) (c) | ||||||||||||||||||
Equity compensation plans approved by security holders
|
|
|
| |||||||||||||||||||||
2020 Long-Term Equity-Based | 33,208 | — | 73,971,666 | (1) | ||||||||||||||||||||
2015 Long-Term Equity-Based
|
| 51,162,412
|
|
| $40.09
|
|
| 99,426,185
| (1)
| 43,609,904 | $ | 31.24 | — | (2) | ||||||||||
2010 Long-Term Equity-Based
|
| 23,760,533
|
|
| $49.07
|
|
|
| (2)
| 12,311,573 | $ | 48.49 | — | (2) | ||||||||||
2008 Employee Stock Purchase Plan For U.S. Employees
|
| —
|
|
| —
|
|
| 4,764,646
| (3)
| — | — | 678,621 | (3) | |||||||||||
Equity compensation plans not approved by security holders
|
| —
|
|
| —
|
|
| —
|
| — | — | — | ||||||||||||
Total
|
|
74,922,945
|
|
|
$44.31
|
|
|
104,190,831
|
(1)
| 55,954,685 | $ | 37.27 | 74,650,287 | (1) | ||||||||||
| (1) | Includes awards that were cancelled or forfeited under the 2010 and 2015 Long-Term Equity-Based Incentive |
| (2) | This plan expired and no future grants are available thereunder. |
| (3) | A total of 8,500,000 shares have been authorized for purchase at a discount |
94 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 85 | |
Certain Relationships and Related Party Transactions
In December 2012, weNovember 2019, Teva entered into two collaborative research agreements with Tel Aviv University pursuant to which Teva will provide funding in the amounts of €250,000 and $94,500, respectively, and will work with the Tel Aviv University scientists to advance cancer and brain studies. These agreements will be extended in the coming months, following finalization of the research plan. Prof. Ronit Satchi-Fainaro, a collaborative developmentmember of our Board of Directors, has been a professor at Tel Aviv University since 2015. Prof. Ronit Satchi-Fainaro holds various other positions at Tel Aviv University, including Head of the Cancer Research and exclusive worldwide license agreement with Xenon for its compound XEN402. XEN402 (now designated by us asTV-45070) targets sodium channels foundNanomedicine Laboratory since 2006, Chair of the Department of Physiology and Pharmacology at the Sackler Faculty of Medicine since 2014, The Kurt and Herman Lion Chair in sensory nerve endings that can increase in chronic painful conditions,Nanosciences and is currently in clinical development for neuropathic pain. Dr. Michael Hayden, who was our PresidentNanotechnologies since 2017, Director of Global R&D and Chief Scientific Officer until November 27, 2017, is a founder, a minority shareholderthe Cancer Biology Research Center since 2020 and a member of the board of directors of Xenon. We paid Xenon an upfront fee of $41 million and may have been required to pay development, regulatory and sales-based milestones of up to $335 million. Xenon was also entitled to royalties on sales and had an option to participate in commercialization in the United States. As required by the agreement, in November 2014, we invested an additional $10 million in Xenon, in connection with its initial public offering. In order to avoid potential conflicts of interest, we have established certain procedures to exclude Dr. Hayden from involvement in Teva’s decision-making related to Xenon. The phase 2 proof of concept study forTV-45070 did not meet primary and secondary endpoints in 2017.Preclinical Dean’s Committee since 2015.
The related party transaction described above was reviewed and approved in accordance with the provisions of the Israeli Companies Law, Teva’s Articles of Association and Teva policy, as described below.
On March 26, 2018, we terminated the collaborative development and exclusive worldwide license agreement by mutual agreement with Xenon. Any extensions to these agreements will also be reviewed accordingly.
Approval of Related Party Transactions
The Israeli Companies Law requires that an “office holder” (as defined in the Israeli Companies Law) of a company promptly disclose any personal interest that he or she may have and all related material information known to him or her, in connection with any existing or proposed transaction of the company.
Pursuant to the Israeli Companies Law, any transaction with an office holder or in which the office holder has a personal interest (other than with respect to such office holder’s Terms of Office and Employment, see “Compensation-Related“Executive Compensation—Compensation Discussion and Analysis—Compensation-Related Requirements of the Israeli Companies Law” above)) must be brought before the Audit Committee, in order to determine whether such transaction is an “extraordinary transaction” (defined as a transaction not in the ordinary course of business, not on market terms or likely to have a material impact on the company’s profitability, assets or liabilities).
Pursuant to the Israeli Companies Law, the Articles of Association and Teva written policy, in the event that the Audit Committee determines that the transaction is not an extraordinary transaction, the transaction will require only Audit Committee approval; if, however, it is determined to be an extraordinary transaction, Board approval is also required and, in some circumstances, shareholder approval may also be required. Such a transaction may only be approved if it is determined to be in the best interests of Teva.
A person with a personal interest in the matter generally may not be present at meetings of the Board or certain committees where the matter is being considered and, if a member of the Board or a committee, may generally not vote on the matter.
Transactions with Controlling Shareholders
Under Israeli law, extraordinary transactions with a controlling shareholder, or in which the controlling shareholder has a personal interest, and any engagement with a controlling shareholder, or a controlling shareholder’s relative, with respect to the provision of services to the company or their Terms of Office and Employment as an office holder or their employment, if they are not an office holder, generally require the approval of the Audit Committee (or with respect to Terms of Office and Employment, the HR and Compensation
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 95
Related Party Transactions
Committee), the Board of Directors and the shareholders. If required, shareholder approval must include (i) at least a majority of the shareholders who do not have a personal interest in the transaction and are present and voting at the meeting (abstentions are disregarded), or, alternatively, that (ii) the total shareholdings of the disinterested shareholders who vote against the transaction do not represent more
| 86 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Related Party Transactions
than two percent of the voting rights in the company. Transactions for a period of more than three years generally need to be brought for approval in accordance with the above procedures every three years.
A shareholder who holds 25% or more of the voting rights in a company is considered a controlling shareholder for these purposes if no other shareholder holds more than 50% of the voting rights. If two or more shareholders are interested parties in the same transaction, their shareholdings are combined for the purposes of calculating percentages.
Change of Control
Subject to certain exceptions, the Israeli Companies Law generally requires that a merger between two Israeli companies be approved by both the board of directors and by the shareholders of each of the merging companies by a simple majority (unless a higher majority is required by the articles of association).
Furthermore, the Israeli Companies Law generally requires that an acquisition of shares in a public company be made by means of a tender offer if as a result of the acquisition the purchaser would hold 25% or more of the voting rights of the company if there is no other holder of 25% or more of the company’s voting rights, or more than 45% of the voting rights of the company if there is no other holder of more than 45% of the company’s voting rights. The Israeli Companies Law generally further requires that such offer be consummated only if at least 5% of the company’s voting rights will be acquired, and that subject to certain exceptions, the majority of the offerees who responded to the offer accepted the offer.
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 87 | |
Shareholder Proposals for the 20182021 Annual Meeting and the 20192022 Annual Meeting
Under Israeli law, one or more shareholders holding 1% or more of the voting rights of Teva may requestpropose to include any matter appropriate for deliberation at a proposalshareholders meeting to be included on the agenda of a shareholders meeting (including proposing the nomination of a candidate to the Board of Directors which will be brought for consideration by Teva’s Corporate Governance and Nominating Committee) by submitting such proposal within seven days of publication of Teva’s notice with respect to its general meeting of shareholders.shareholders, unless Teva publishes a preliminary notice at least twenty-one days prior to a publication of the notice of the meeting, stating its intention to convene such meeting and the agenda thereof, in which case the shareholder proposal should be submitted within fourteen days of such preliminary notice. Accordingly, any such shareholder holding 1% or more of the voting rights of Teva may request to include a proposal on the agenda of thisthe Annual Meeting by submitting such proposal in writing to Teva no later than May 2, 2018,April 28, 2021, at its executive offices at 5 Basel124 Dvora HaNevi’a Street, P.O. Box 3190, Petach Tikva 4951033,Tel Aviv, 6944020, Israel, Attn: Dov Bergwerk, Company Secretary.
Under Teva’s Articles of Association, one or more shareholders holding 1% or more of the voting rights in Teva (or a shareholder interested in proposing the nomination of certain candidate(s) for election as director(s) for consideration by the Corporate Governance and Nominating Committee) may propose to include a matter on the agenda of the 20192022 annual meeting of shareholders by submitting the proposal in writing to Teva at its executive offices at 5 Basel124 Dvora HaNevi’a Street, P.O. Box 3190, Petach Tikva 4951033,Tel Aviv, 6944020, Israel, Attn: Dov Bergwerk, Company Secretary, no later than 14 days after the date of first publication by Teva of its 20182021 consolidated financial statements.
Any such shareholder proposal must comply with the requirements of applicable law and Teva’s Articles of Association. The requirements under Teva’s Articles of Association include providing information such as: (i) the number of ordinary shares held by the proposing shareholder, directly or indirectly, and, if any such ordinary shares are held indirectly, an explanation of how they are held and by whom; (ii) the shareholder’s purpose in making the request; (iii) any agreements, arrangements, understandings or relationships between the shareholder and any other person with respect to any securities of Teva or the subject matter of the request; and (iv) if the shareholder wishes to include a statement in support of his or her proposal in Teva’s proxy statement, if provided or published, a copy of such statement. If the proposal is to nominate a candidate for election to the Board of Directors, the proposing shareholder must also provide (a) a declaration signed by the nominee and any other information required under the Israeli Companies Law, (b) additional information in respect of the nominee as would be required in response to the applicable disclosure requirements in Israel or abroad, including the information responsive to Items 401, 403 and 404 of RegulationS-K under the U.S. Securities Act, of 1933, as amended, to the extent applicable, (c) a representation made by the nominee of whether the nominee meets the objective criteria for an independent director of a company such as Teva under any applicable law, regulation or stock exchange rules, in Israel or abroad, and if not, then an explanation of why not, and (d) details of all relationships and understandings between the proposing shareholder and the nominee.
Under Rule14a-8 of the Exchange Act, a shareholder proposal to be included in the proxy statement and proxy card for the 20192022 annual general meeting of shareholders pursuant to Rule14a-8 must be received at our principal office on or before December 26, 201831, 2021 and must comply withRule 14a-8.
96 Teva Pharmaceutical Industries Ltd.2018 Proxy Statement
| 88 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Related Party Transactions
In accordance with SEC rules, notwithstanding anything to the contrary set forth in any of our previous or future filings under the U.S. Securities Act of 1933, as amended, or the Exchange Act that might incorporate this proxy statementProxy Statement or future filings made by Teva under those statutes, the information included under the caption “Compensation“HR and Compensation Committee Report” and those portions of the information included under the caption “Audit Committee Report” required by the SEC’s rules to be included therein shall not be deemed to be “soliciting material” or “filed” with the SEC and shall not be deemed incorporated by reference into any of those prior filings or into any future filings made by the CompanyTeva under those statutes, except to the extent we specifically incorporate these items by reference.
Householding of Proxy Materials
Some banks, brokers and other nominee record holders may participate in the practice of “householding” proxy statements. This means that only one copy of the proxy materials may have been sent to multiple shareholders in your household. Teva will promptly deliver a separate copy of the proxy statement, as well as its annual report,Annual Report, to you if you write to or call Teva at the following addressCompany at: TevaIR@tevapharm.com or phone numbers:mailing address: Teva Pharmaceutical Industries Ltd., 5 Basel124 Dvora HaNevi’a Street, Petach Tikva,Tel Aviv, 6944020, Israel, phone: +972(3) 926-7656, Attn: Investor Relations, or in the United States at +1phone: +972 (215) 591-8912.(3) 914-8262. If you want to receive copies of Teva’s proxy statement in the future, or if you are receiving multiple copies and would like to receive only one copy for your household, you should contact your bank, broker or other nominee record holder, or you may contact us at the above address and phone numbers.
* * *
Teva Pharmaceutical Industries Ltd. 2018 Proxy Statement 97
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 89 | |
Appendix AQuestions and Answers about the Annual Meeting
TEVA PHARMACEUTICAL INDUSTRIES LIMITEDThe Meeting
2008 Employee Stock Purchase PlanWhen and how will the Annual Meeting be held?
for U.S. EmployeesThe Annual Meeting will be conducted in a virtual format on Monday, June 14, 2021, at 4:00 p.m., Israel time, 9:00 a.m., Eastern time.
As amended and restated effective September 7, 2017
1. Purpose. The purposeIn the interest of the Plan is to providehealth and safety of our shareholders, directors, officers and employees, of each Designated Employer with an opportunity to purchase ADSs, of Teva Pharmaceutical Industries Limited through accumulated payroll deductions. It is the intentionin light of the Issuer,ongoing COVID-19 pandemic, shareholders will not be able to physically attend the CompanyAnnual Meeting.
For additional information on how to vote and participate at the other Designated EmployersAnnual Meeting see below under “Participating and Voting at the Annual Meeting.”
Who may attend the Annual Meeting?
Attendance at the Annual Meeting, including any adjournments or postponements thereof, will be limited to have the Plan qualifyholders of record as an “employee stock purchase plan” under Section 423 of the Code. The provisionsclose of the Plan, accordingly, shall be construed so as to extend and limit participation in a manner consistent with the requirements of that section of the Code.
2. Definitions.
(a)business on May 5, 2021 (the “ADSsRecord Date” shall mean the) who hold ordinary shares or American Depositary Shares (“ADSs”), directly in their own name, and beneficial owners who hold ordinary shares or ADSs through a broker, bank or other nominee rather than directly in their own name, and each of their legal proxy holders or their authorized persons.
What is a quorum for the Annual Meeting?
A minimum of two holders of ordinary shares (or ADSs representing such ordinary shares) who are present at the Annual Meeting, in person or by proxy or represented by their authorized persons, and who hold in the aggregate twenty-five percent or more of such ordinary shares (or ADSs representing such ordinary shares), will constitute a legal quorum. At the close of business on April 1, 2021, 1,102,349,072 ordinary shares were outstanding and entitled to vote. Ordinary shares held in treasury will not be included in the calculation to determine if a quorum is present. Abstentions and broker non-votes will be considered present and entitled to vote for the purpose of determining the presence of a quorum. Should no legal quorum be present one half hour after the scheduled time, the Annual Meeting will be adjourned to one week from that day, at the same time and in virtual format. Should such legal quorum not be present one half hour after the time set for the Annual Meeting, as adjourned, any two holders of ordinary shares present, in person or by proxy, who jointly hold twenty percent or more of such ordinary shares (or ADSs representing ordinary shares) will then constitute a legal quorum.
Who may vote at the Annual Meeting?
Ordinary Shares
Holders of record of ordinary shares as of the Issuer, each representing one Ordinary ShareRecord Date may vote at the Annual Meeting.
Beneficial owners who hold ordinary shares through a nominee company pursuant to Section 177(1) of the Issuer.
(b) “Code” shall mean the United States Internal Revenue Code of 1986,Israeli Companies Law, as amended.
(c) “Committee” shall mean the Compensation Committee of the Company BoardRecord Date (a “Non-Registered Holder”), rather than directly in their own name, have the right to direct their broker, bank or an administrative committee appointedother nominee how to vote using the instructions provided by the Issuer Board,broker, bank or other nominee, but may not vote their shares at the Annual Meeting unless they obtain a proof of share ownership as of the Record Date (“Proof of Ownership”), which shallmust be approved by a member of the administrative committeeTel Aviv Stock Exchange (“TASE”).
ADSs
As an ADS holder, you will not be entitled to vote at the Annual Meeting through the online meeting platform. To the extent you provide the Depositary (as defined below) or your broker, bank or other
| 90 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Questions and Answers about the Annual Meeting
nominee, as applicable, with voting instructions, the Depositary has advised us that it will vote the ordinary shares underlying your ADSs in accordance with your instructions.
You also may exercise the right to vote the ordinary shares underlying your ADSs by surrendering your ADSs to Citibank, N.A., as depositary for the Plan.
(d)ADSs (the “CompanyDepositary” shall mean Teva Pharmaceuticals USA, Inc.
(e) “Company Board” shall mean the Board of Directors) for cancellation and withdrawal of the Company.
(f)corresponding ordinary shares pursuant to the terms described in the Second Amended and Restated Deposit Agreement (the “CompensationDeposit Agreement” shall mean all wages, salary, overtime, bonuses,), dated as of December 4, 2018, by and commissions.
(g) “Designated Employer” shall mean each United States Subsidiary.
(h) “Employee” shall mean any individual who is an employeeamong the Company, the Depositary, and the holders and beneficial owners of any Designated Employer for purposes of tax withholding underADSs. In order to be able to vote at the Code. For purposesAnnual Meeting, you must complete the ADS cancellation process and become a holder of the Plan,corresponding ordinary shares by the employment relationshipRecord Date. However, it is possible that you may not have sufficient time to withdraw your ordinary shares and vote them at the upcoming Annual Meeting as a holder of record of ordinary shares as of the Record Date. Holders of ADSs may incur additional costs associated with the ADS cancellation process.
The Israeli Companies Law does not permit shareholders of public companies to act by written consent.
Participating and Voting at the Annual Meeting
How can I access the online meeting platform and vote my ordinary shares or ADSs?
To access the Annual Meeting, holders of Teva’s ADSs and ordinary shares should visit www.meetingcenter.io/212475968 and, when prompted, enter their control number (as described below). The meeting password is TEVA2021.
Your vote is very important and we encourage you to vote your shares and submit your proxy regardless of whether or not you plan to attend the Annual Meeting. Each issued and outstanding ordinary share (or ADS representing an ordinary share) shall entitle its holder to one vote on each matter properly submitted at the Annual Meeting. Ordinary shares held in treasury by Teva do not entitle Teva to vote in respect thereof at the Annual Meeting.
Ordinary Shares
Record holders of ordinary shares: If you are the record holder of ordinary shares as of the Record Date, you have the right to (i) vote at the Annual Meeting through the online meeting platform, (ii) vote by submitting your proxy card by mail or by email to TevaAGM2021@tevapharm.com, or (iii) grant your voting proxy to an authorized person.
If you choose to submit your proxy card by mail or by email to TevaAGM2021@tevapharm.com, mark the enclosed proxy card in accordance with the instructions, date, sign and return it to Teva. To be treatedtaken into account, your proxy card must be received by Teva, by 4:00 p.m., Israel time, on June 10, 2021, unless determined otherwise by the chairman of the Annual Meeting.
In order to access the Annual Meeting online platform, record holders of ordinary shares must enter a control number. The control number will consist of the holder’s Israeli identification number or Israeli company registration number as continuing intact whileit appears in Teva’s share register. Holders of Teva’s ordinary shares as of the individual is on sick leaveRecord Date will also be able to ask questions through the online meeting platform, which will be open for questions 24 hours prior to and throughout the Annual Meeting.
If you appoint another person to act as your authorized proxy, such proxy must be submitted in writing by mail or other leave of absenceby email to TevaAGM2021@tevapharm.com (in a form approved by the Designated Employer,Company Secretary) from the record holder of ordinary shares, including such record holder’s Israeli identification number or company registration number, along with the authorized proxy’s name, Israeli identification number, proof of identification and email address, and must be received by Teva by 4:00 p.m., Israel time, on June 10, 2021, unless determined otherwise by the chairman of the Annual Meeting. The submission of a proxy by
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 91 | |
Questions and Answers about the Annual Meeting
the aforementioned deadline will also serve to register the authorized proxy in advance in order to participate in the Annual Meeting, vote your shares and ask questions through the online meeting platform. Such authorized proxy will then be able to use their Israeli identification number as applicable. Whereit appears on the periodproof of leave exceeds 90 daysIsraeli identification provided to Teva as the control number to access the online platform.
Non-registered holders of ordinary shares: If you are a Non-Registered Holder, you may (i) direct your broker, bank or other nominee how to vote using the instructions provided by such broker, bank or other nominee; (ii) vote through the electronic voting system of the Israeli Securities Authority at least six hours prior to the Annual Meeting (i.e., before 10:00 a.m., Israel time, on June 14, 2021); or (iii) if you obtain a Proof of Ownership as detailed above, submit your vote (a) by submitting your proxy card by mail or by email to TevaAGM2021@tevapharm.com, together with the Proof of Ownership, by 4:00 p.m., Israel time, on June 10, 2021, unless determined otherwise by the chairman of the Annual Meeting; or (b) vote at the Annual Meeting through the online meeting platform.
In order to access the online meeting platform, Non-Registered Holders of ordinary shares must register in advance to participate in the Annual Meeting, vote their shares and ask questions through the online meeting platform, which will be open for questions 24 hours prior to and throughout the Annual Meeting.
To register, Non-Registered Holders must submit the Proof of Ownership (including Israeli identification number or company registration number) reflecting the number of ordinary shares beneficially owned as of the Record Date, along with their name and email address, to Teva at TevaAGM2021@tevapharm.com. Requests for registration must be received no later than June 10, 2021 at 4:00 p.m., Israel time.
Non-Registered Holders will then be able to use their Israeli identification number or company registration number as it appears on the Proof of Ownership provided to Teva as the control number to access the online platform. If a Non-Registered Holder would like to appoint another person to act as his/her authorized proxy, such proxy must be submitted to Teva along with the Proof of Ownership, following the instructions set forth above.
ADSs
The Deposit Agreement sets out the rights of ADS holders as well as the rights and obligations of the Depositary. Each ADS represents the right to receive one ordinary share deposited with Citibank Tel Aviv, as custodian for the Depositary under the Deposit Agreement or any successor custodian.
Record holders of ADSs: If you are a record holder of ADSs as of the Record Date, you will receive instructions from the Depositary for the ordinary shares underlying your ADSs to be voted. If you held ADSs directly as of the Record Date, you have the right to instruct the Depositary how to vote. So long as the Depositary receives your voting instructions by 8:00 a.m., Eastern time, on June 11, 2021, it will, to the extent practicable and subject to Israeli law and the individual’s rightterms of the Deposit Agreement, vote the underlying ordinary shares as you instruct.
Record holders of ADSs as of the close of the Record Date will be able to reemployment isparticipate in the Annual Meeting and ask questions through the online meeting platform, which will be open for questions 24 hours prior to and throughout the Annual Meeting; however, they may not guaranteed eithervote through the online meeting platform.
To access the online meeting platform, record holders of ADSs should enter the 15-digit control number on the proxy card or Notice of Internet Availability of Proxy Materials received.
Beneficial owners of ADSs that are registered in the name of a broker, bank or other agent: If you beneficially own ADSs as of the Record Date through a broker, bank or other nominee, such intermediary will provide you instructions on how you may vote the ordinary shares underlying your ADSs. Please check with your broker, bank or other nominee, as applicable, and carefully follow the voting procedures provided to you.
| 92 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Questions and Answers about the Annual Meeting
To participate in the Annual Meeting and ask questions through the online meeting platform, which will be open for questions 24 hours prior to and throughout the Annual Meeting, beneficial holders of ADSs that are registered in the name of a broker, bank or other agent must register in advance; however, they may not vote through the online meeting platform.
To register, beneficial holders of ADSs must submit proof of ownership reflecting the number of ADSs beneficially owned as of the Record Date, along with their name and email address, to Computershare at legalproxy@computershare.com. Requests for registration must be labeled as “TEVA MEETING REQUEST” and must be received no later than Friday, June 11, 2021 at 8:00 a.m., Eastern time. Beneficial holders of ADSs will then receive a confirmation of registration with a 15-digit control number by statuteemail from Computershare.
How will my ordinary shares or ADSs be voted if I do not vote?
Ordinary Shares
If you hold ordinary shares and do not (i) vote at the Annual Meeting through the online meeting platform, (ii) vote by submitting your proxy card by mail or by contract,email, (iii) grant your voting proxy to an authorized person or (iv) as a Non-Registered Holder, vote by submitting your proxy card and proof of ownership by mail, by email or through the employment relationshipelectronic voting system of the Israeli Securities Authority, your ordinary shares will not be deemed tocounted as votes cast and will have terminatedno effect on the 91st day of such leave.
(i) “Enrollment Date” shall mean the first Trading Day of each Offering Period.
(j) “Exchange Act” shall mean the United States Securities Exchange Act of 1934, as amended.
(k) “Exercise Date” shall mean the last Trading Day of each Offering Period.
(l) “Fair Market Value” shall mean, as of any date, the valueoutcome of the vote with respect to any matter.
ADSs
If you are a record holder of ADSs determinedand do not instruct the Depositary how to vote the ordinary shares underlying your ADSs, the ordinary shares underlying your ADSs will not be counted as follows:votes cast and will have no effect on the outcome of the vote with respect to any matter.
(i) If theyou are a beneficial owner whose ADSs are listed on any established stock exchange orheld of record by a national market system, including without limitationbroker, your broker has “discretionary voting” authority under the New York Stock Exchange (“NYSE”NYSE”), rules to vote the Fair Market Value shall beshares represented by your ADSs on “routine” matters, such as the averageratification of the high and low sale price for the ADSs (or the averageappointment of the closing bid and asked prices, if no sales were reported)Kesselman & Kesselman, a member of PricewaterhouseCoopers International Ltd., as quoted on such exchange (orour independent registered public accounting firm, even if the exchange with the greatest volume of trading in ADSs) or systembroker does not receive voting instructions from you. However, your broker does not have discretionary authority absent specific instructions from you to vote on the datefollowing “non-routine” matters: the election of such determination, if such date is a Trading Day, or if such date is not a Trading Day, thendirectors and the advisory vote on the Trading Day immediately preceding such date, as reportedcompensation of our named executive officers, in The Wall Street Journal or such other source as the Company Board deems reliable; or
(ii) If the ADSs are quoted on the NYSE or are regularly quoted bywhich case a recognized securities dealer but selling prices are not reported, the Fair Market Value shall be the average of the closing bid and asked prices for the ADSs on the date of such determination, if such date is a Trading Day, or if such date is not a Trading Day, then on the Trading Day immediately preceding such date, as reported in The Wall Street Journal or such other source as the Company Board deems reliable; or
(iii) In the absence of an established market for the ADSs, the Fair Market Value thereof shall be determined in good faith by the Company Board.
(m) “Issuer” shall mean Teva Pharmaceutical Industries Limited.
(n) “Issuer Board” shall mean the Board of Directors of the Issuer.
(o) “Offering Period” shall mean, subject to the second sentence of Section 4 hereof, each quarter commencing on the first Trading Day on or after January 1, April 1, July 1, and October 1 ending on or prior to the following March 31, June 30, September 30, and December 31, respectively.
(p) “Ordinary Shares” shall mean the ordinary shares of the Issuer, NIS 0.1 par value per share.
(q) “Parent” shall mean a corporation which is a “parent corporation” of the Issuer within the meaning of section 424(e) of the Code.
(r) “Plan” shall mean this Teva Pharmaceutical Industries Limited 2008 Employee Stock Purchase Plan for U.S. Employees, as amended.
(s) “Purchase Price” shall mean an amount equal to 85% of the Fair Market Value of an ADS on the Exercise Date, provided that, effective with the first Offering Period commencing on January 1, 2016 the Purchase Price shall be 95% of the Fair Market Value of an ADS on the Exercise Date.
(t) “Reserves” shall mean the number of ADSs covered by each option under the Plan which have not yet been exercisedbroker non-vote will occur and the number ofshares represented by your ADSs which have been authorized for issuance under the Plan but not yet placed under option.
(u) “Subsidiary” shall mean a corporation which is a “subsidiary corporation” of the Issuer within the meaning of section 424(f) of the Code.
(v) “Trading Day” shall mean a day on which national stock exchanges and NYSE (as defined above) are open for trading.
3. Eligibility.
(a) Each person who is an Employee, on a given Enrollment Date, shall be eligible to participate in the Plan.
(b) Any provisions of the Plan to the contrary notwithstanding, no Employee shall be granted an option under the Plan (i) if, immediately after the grant, such Employee would own the ADSs (together with stock owned by any other person or entity that would be attributed to such Employee pursuant to section 424(d) of the Code) of the Issuer (including, for this purpose, all shares of stock subject to any outstanding options to purchase such stock, whether or not currently exercisable and irrespective of whether such options are subject to the favorable tax treatment of section 421(a) of the Code) possessing five percent (5%) or more of the total combined voting power or value of all classes of stock of the Issuer or of any Parent or Subsidiary, or (ii) which permits his or her rights to purchase stock under all employee stock purchase plans (within the meaning of section 423 of the Code) of the Issuer and its Parents and Subsidiaries to accrue at a rate which exceeds Twenty-Five Thousand Dollars ($25,000) worth of the ADSs
(determined at the Fair Market Value of the ADSs at the time such option is granted) for each calendar year in which such option is outstanding at any time. The limitation described in clause (ii) of the preceding sentence shall be applied in a manner consistent with Section 423(b)(8) of the Code.
4. Offering Periods. The Plan shall be implemented by consecutive Offering Periods continuing from the first Offering Period until terminated in accordance with Section 18 hereof. The Company Board shall have the power to change the duration of Offering Periods (including the commencement dates thereof) with respect to future offerings without shareholder approval if such change is announced at least twenty-five (25) days prior to the scheduled beginning of the first Offering Period to be affected thereafter.
5. Participation.
(a) An Employee may become a participant in the Plan for an Offering Period by completing a subscription agreement authorizing payroll deductions in and submitting it to the Committee (or such person as may be designated by the Committee from time to time) no later than the 20th day of the month immediately prior to the applicable Enrollment Date, unless a later time for submitting the subscription agreement is set by the Company Board for all Employees with respect to a given Offering Period. An Employee may complete and submit a subscription agreement in any manner, including electronically, as the Committee may prescribe from time to time. By submitting a subscription agreement, the Employee agrees to be bound by the terms of the Plan.
(b) Payroll deductions for a participant shall commence on the first payroll date on or following the Enrollment Date and shall end on the last payroll date in the Offering Period to which such authorization is applicable, unless sooner terminated by the participant as provided in Section 10 hereof.
6. Payroll Deductions.
(a) At the time a participant submits his or her subscription agreement, he or she shall elect to have payroll deductions made on each pay day during the Offering Period in an amount (expressed as a whole number percentage) not exceeding ten percent (10%) of the Compensation which he or she receives on each pay day during the Offering Period; provided, however, that the maximum number of ADSs which may be purchased by any participant during any Offering Period is the number of ADSs equal to (i) $25,000 minus the Fair Market Value of the number of ADSs previously purchased during such calendar year, such Fair Market Value determined as of each such prior Enrollment Date during the calendar year with respect to which the ADSs were previously purchased, divided by (ii) the Fair Market Value of the ADSs on the Enrollment Date for the current Offering Period.
(b) All payroll deductions made for a participant shall be credited to his or her account under the Plan and will be withheld in whole percentages only. A participant may not make any additional payments into such account.
(c) A participant may discontinue his or her participation in the Plan, as provided in Section 10 hereof, at any time during the Offering Period prior to the Exercise Date. Once an Offering Period has commenced, a participant may not increase or decrease the rate of his or her payroll deductions for that Offering Period, but may, during that Offering Period, increase or decrease the rate of his or her payroll deductions for the next succeeding Offering Period, by completing or submitting with the Committee (or such person as may be designated by the Committee from time to time) a new subscription agreement, no later than the 20th day of the month immediately prior to the end of that Offering Period, authorizing a change in payroll deduction rate. A participant’s subscription agreement shall remain in effect for successive Offering Periods unless terminated as provided in Section 10 hereof.
(d) Notwithstanding the foregoing, a participant’s payroll deductions may be decreased by the Committee to 0% (i) at any time, to the extent necessary to comply with Section 423(b)(8) of the Code and Section 3(b)
hereof, and (ii) for each Offering Period, at such time during such Offering Period that the aggregate Fair Market Value of the ADSs (measured as of the date of each respective Enrollment Date) previously purchased when added to the Fair Market Value of the ADSs to be purchased with respect to such then current Offering Period equals or would exceed $25,000 in such calendar year. Subject to the preceding sentence, payroll deductions shall recommence at the rate provided in such participant’s subscription agreement at the beginning of the next succeeding Offering Period, unless terminated by the participant as provided in Section 10 hereof.
(e) At the time the option is exercised, in whole or in part, or at the time some or all of the ADSs issued under the Plan are disposed of, the participant must make adequate provisions for the Designated Employer’s federal, state, or other tax withholding obligations, if any, which arise upon the exercise of the option or the disposition of the ADSs. At any time, the Designated Employer may, but will not be obligatedvoted on these matters.
What are the voting requirements to withhold fromelect the participant’s compensation the amount necessary for the Designated Employerdirectors and to meet applicable withholding obligations, including any withholding required to make available to the Designated Employer any tax deductions or benefits attributable to sale or early dispositionapprove each of the ADSs by the Employee.proposals discussed in this Proxy Statement?
7. GrantThe affirmative vote of Option. On the Enrollment Date of each Offering Period, each Employee participating in such Offering Period shall be granted an option to purchase on the Exercise Date of such Offering Period (at the applicable Purchase Price) up to a number of ADSs determined by dividing such Employee’s payroll deductions accumulated prior to such Exercise Date and retained in the participant’s account as of the Exercise Date by the applicable Purchase Price; provided, however, that the maximum number of ADSs which may be purchased by any participant during any Offering Period is the number of ADSs equal to (i) $25,000 minus the Fair Market Value of the number of ADSs previously purchased during such calendar year, such Fair Market Value determined as of each such prior Enrollment Date during the calendar year with respect to which such shares were previously purchased, divided by (ii) the Fair Market Value of the ADSs on the Enrollment Date for the current Offering Period, and provided, further, that such purchase shall be subject to the limitations set forth in Sections 3(b) and 12 hereof. Exercise of the option shall occur as provided in Section 8 hereof, unless the participant has withdrawn pursuant to Section 10 hereof, and shall expire on the last day of the Offering Period.
8. Exercise of Option. Unless a participant withdraws from the Plan as provided in Section 10 hereof, his or her option for the purchase of ADSs will be exercised automatically on the Exercise Date, and, subject to the limitations set forth in Sections 3(b), 7 and 12 hereof, the maximum number of full and fractional ADSs subject to option shall be purchased for such participant at the applicable Purchase Price with the accumulated payroll deductions in his or her account. During a participant’s lifetime, a participant’s option to purchase ADSs hereunder is exercisable only with respect to such participant.
9. Crediting of ADSs and Delivery or Sale of ADSs. As promptly as practicable after each Exercise Date on which a purchase of ADSs occurs, the Company (or the applicable Designated Employer) shall arrange for the full and fractional portion of the ADSs to be deposited in the brokerage account of each participant at a brokerage house designated by the Committee. The ADSs shall be held in such brokerage account until such time as the participant, or his or her designated beneficiary or estate in the event of a participant’s death, requests delivery of a certificate representing any of the ADSs or requests that any ADSs be sold and the proceeds therefrom be distributed to such participant; provided, however, that, for the avoidance of doubt, no ADSs were withdrawn or sold prior to such time as the Plan was initially approved by the shareholders of the Issuer holding a majority of its outstanding ADSs and Ordinary Shares. Upon the request of a participant, or his or her designated beneficiary or estate in the event of a participant’s death, any fractional ADSs will be distributed in cash in the form of a check having a value equal to the value of such fractional ADSs.
10. Withdrawal; Termination of Employment.
(a) A participant may withdraw all but not less than all the payroll deductions credited to his or her account and not yet used to exercise his or her option under the Plan at any time prior to the Exercise Date of an Offering Period by giving written notice to the Committee (or such person as may be designated by the Committee from time to time) in the manner as the Committee may prescribe from time to time. All of the participant’s payroll deductions credited to his or her account will be paid to such participant promptly after receipt of notice of withdrawal and such participant’s option for the Offering Period will be automatically terminated, and no further payroll deductions for the purchase of ADSs will be made during the Offering Period. If a participant withdraws from the Plan during an Offering Period, he or she may not resume participation until the next Offering Period. He or she may resume participation for any other Offering Period by delivering to the Committee (or such person as may be designated by the Committee from time to time) a new subscription agreement no later than the 20th day of the month immediately prior to the Enrollment Date for such Offering Period.
(b) Upon a participant’s ceasing to be an Employee, for any reason, he or she will be deemed to have elected to withdraw from the Plan and the payroll deductions credited to such participant’s account during the Offering Period but not yet used to exercise the option will be distributed to such participant or, in the case of his or her death, to his or her estate, and such participant’s option will be automatically terminated. Unless otherwise determined by the Committee, any full or fractional ADSs held in the brokerage account of such participant shall remain in such account until such participant or, in the case of his or her death, his or her estate, requests that a certificate representing the full ADSs be distributed or that such ADSs be sold and the proceeds from the sale distributed to the participant, or such other person. Upon a participant’s request, any fractional ADSs will be distributed in cash in the form of a check having a value equal to the value of such fractional ADSs; provided, however, that, in the discretion of the Committee, the participant may be responsible for any fees associated with the maintenance of his or her account following such termination of employment.
(c) A participant’s withdrawal from an Offering Period will not have any effect upon his or her eligibility to participate in any similar plan which may hereafter be adopted by the Issuer, the Company or any other Designated Employer.
11. Interest. No interest or other increment shall accrue or be payable with respect to any of the payroll deductions of a participant in the Plan.
12. Stock.
(a) The maximum number of ADSs which shall be made available for sale under the Plan shall be 8,500,000, subject to adjustment upon changes in capitalization of the Issuer as provided in Section 17 hereof. The ADSs granted pursuant to the Plan may be (i) authorized but unissued ADSs, (ii) authorized and issued ADSs held by the Issuer, the Company or any other of the Issuer’s subsidiaries, or (iii) acquired by the Issuer, the Company or any other of the Issuer’s subsidiaries for the purposes of the Plan. If on a given Exercise Date the number of ADSs with respect to which options are to be exercised exceeds the number of ADSs then available under the Plan, the Committee shall make a pro rata allocation of the ADSs remaining available for purchase in as uniform a manner as shall be practicable and as it shall determine to be equitable.
(b) No participant will have an interest or voting right in ADSs covered by his option until such option has been exercised. All ADSs held in a participant’s brokerage account on behalf of a participant shall be voted by such participant. Dividends accruing on the ADSs, if any, held in a participant’s brokerage account shall be reinvested in ADSs at the full market value of such ADSs at the time of purchase and deposited in such brokerage account until such time as the participant requests delivery or sale of the ADSs as set forth in Section 9 herein.
(c) ADSs to be deposited into a participant’s brokerage account under the Plan will be registered in the name of the participant.
13. Administration. The Plan shall be administered by the Committee. The Committee shall have full and exclusive discretionary authority to construe, interpret and apply the terms of the Plan, to determine eligibility and to adjudicate all disputed claims filed under the Plan. The Committee shall also have authority to develop, amend and terminate rules governing the operation of the Plan in conformity with the terms of the Plan. Every finding, decision and determination made by the Committee shall, to the full extent permitted by law, be final and binding upon all parties.
14. Transferability. Neither payroll deductions credited to a participant’s account nor any rights with regard to the exercise of an option or to receive ADSs under the Plan may be assigned, transferred, pledged or otherwise disposed of in any way (other than by will, the laws of descent and distribution) by the participant. Any such attempt at assignment, transfer, pledge or other disposition shall be without effect, except that the Committee may treat such act as an election to withdraw funds from an Offering Period in accordance with Section 10 hereof.
15. Use of Funds. All payroll deductions received or held by any Designated Employer under the Plan may be used by such Designated Employer for any corporate purpose, and the Designated Employer shall not be obligated to segregate such payroll deductions.
16. Reports. Individual accounts will be maintained for each participant in the Plan. Statements of account will be given to participating Employees at least annually, within such time as the Committee may reasonably determine, which statements will set forth the amounts of payroll deductions, the Purchase Price, the number of full and fractional ADSs purchased and held in the participant’s brokerage account.
17. Adjustments Upon Changes in Capitalization.
(a) Changes in Capitalization. Subject to any required action by the shareholders of the Issuer, the Reserves as well as the price per ADS covered by each option under the Plan which has not yet been exercised shall be proportionately adjusted for any increase or decrease in the number of issued ADSs resulting from a stock split, reverse stock split, stock dividend, combination or reclassification of the ADSs, or any other increase or decrease in the number of ADSs or Ordinary Shares effected without receipt of consideration by the Issuer; provided, however, that conversion of any convertible securities of the Issuer shall not be deemed to have been “effected without receipt of consideration.” Such adjustment shall be made by the Issuer Board, whose determination in that respect shall be final, binding and conclusive. Except as expressly provided herein, no issuance by the Issuer of shares of stock of any class, or securities convertible into shares of stock of any class, shall affect, and no adjustment by reason thereof shall be made with respect to, the number or price of ADSs subject to an option.
(b) Dissolution or Liquidation. In the event of the proposed dissolution or liquidation of a Designated Employer or the Issuer, the Offering Period will terminate immediately prior to the consummation of such proposed action, unless otherwise provided by the Issuer Board.
(c) Merger or Asset Sale. In the event of a proposed sale of all or substantially all of the assets of the Issuer, or the merger of the Issuer with or into another corporation, each option under the Plan shall be assumed or an equivalent option shall be substituted by such successor corporation or a parent or subsidiary of such successor corporation, unless the Issuer Board determines, in the exercise of its sole discretion and in lieu of such assumption or substitution, to shorten the Offering Period then in progress by setting a new Exercise Date (the “New Exercise Date”). If the Issuer Board shortens the Offering Period then in progress in lieu of assumption or substitution in the event of a merger or sale of assets, the Issuer Board shall notify each participant in writing, at least ten (10) business days prior to the New Exercise Date, that the Exercise Date for his or her option has been changed to the new Exercise Date and that his or her option will be exercised automatically on the new Exercise Date, unless prior to such date he or she has withdrawn from
the Offering Period as provided in Section 10 hereof. For purposes of this paragraph, an option granted under the Plan shall be deemed to be assumed if, following the sale of assets or merger, the option confers the right to purchase, for each ADS subject to the option immediately prior to the sale of assets or merger, the consideration (whether stock, cash or other securities or property) received in the sale of assets or merger by holders of the ADSs for each ADS held on the effective date of the transaction (and if such holders were offered a choice of consideration, the type of consideration chosen by the holders of a majority of Teva ordinary shares participating and voting at the outstanding ADSs and Ordinary Shares); provided, however, that if such consideration receivedAnnual Meeting, in the sale of assetsperson or merger was not solely common stockby proxy or through their representatives, is required to adopt each of the successor corporation or its parent (as defined in Section 424(e)proposals. Cumulative voting is not permitted. Under the terms of the Code),Deposit Agreement, the Issuer Board may,Depositary shall endeavor (insofar as is practicable and in accordance with our Articles of Association) to vote or cause to be voted the number of ordinary shares represented by ADSs in accordance with the consentinstructions provided by the holders of the successor corporation and the participant, provide for the consideration to be received upon exercise of the option to be solely common stock of the successor corporation or its parent equal in Fair Market ValueADSs to the per share considerationDepositary by the deadline set. If instructions are not received by holders of the ADSs in the sale of assets or merger.
The Issuer Board may, if it so determines in the exercise of its sole discretion, also make provision for adjusting the Reserves, as well as the price per ADS covered by each outstanding option, in the event the Issuer effects one or more reorganizations, recapitalizations, rights offerings or other increases or reductions of its outstanding ADSs or Ordinary Shares, and in the event of the Issuer being consolidated with or merged into any other corporation.
18. Amendment or Termination.
(a) The Issuer Board may at any time and for any reason terminate or amend the Plan. Except as provided in Section 17 hereof, no such termination may adversely affect options previously granted; provided, that an Offering Period may be terminatedDepositary by the Issuer Board on any Exercise Date ifdeadline, the Issuer Board determines thatordinary shares represented by such uninstructed ADSs shall not be voted at the termination of the Plan is in the best interests of the IssuerAnnual Meeting. If instructions are signed and its shareholders. Except as provided in Section 17 hereof, no amendment may make any change in any option theretofore granted which adversely affects the rights of any participant. To the extent necessary to comply with Section 423 of the Code (or any successor rule or provision or any other applicable law or regulation), the Issuer shall obtain shareholder approval in such a manner and to such a degree as required.
(b) Without shareholder consent and without regard to whether any participant rights may be considered to have been “adversely affected,” the Company Board (or the Committee) shall be entitled to change the Offering Periods, limit the frequency or number of changes in the amount withheld during an Offering Period, establish the exchange ratio applicable to amounts withheld in a currency other than U.S. dollars, permit payroll withholding in excess of the amount designated by a participant in order to adjust for delays or mistakes in a Designated Employer’s processing of properly completed withholding elections, establish reasonable waiting and adjustment periods or accounting and crediting procedures to ensure that amounts applied toward the purchase of the ADSs for each participant properly correspond with amounts withheld from the participant’s Compensation, and establish such other limitations or procedures as the Company Board (or the Committee) finds, in its sole discretion, advisable and consistent with the Plan.
19. Notices. All notices or other communications by a participanttimely returned to the Company orDepositary, but no specific voting instruction is marked for a proposal, the Committee under or in connection with the Planholder shall be deemed to have been duly given when received indirected the form specified byDepositary to give voting instructions “FOR” the Company or the Committee at the location, or by the person, designated by the Company or the Committee for the receipt thereof.
20. Conditions Upon Sale of ADSs. ADSs shall not be sold with respect to an option unless the exercise of such option and the sale of such ADSs or the underlying Ordinary Shares pursuant thereto shall comply with all applicable provisions of law, domestic or foreign, including, without limitation, the United States Securities Act of 1933, as amended, the Exchange Act, the rules and regulations promulgated thereunder and the requirements of any stock exchange upon which the ADSs or Ordinary Shares may then be listed, and shall be further subject to the approval of counsel for the Company or the Issuer with respect to such compliance.unmarked proposal.
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 93 | |
Questions and Answers about the Annual Meeting
21. RepresentationsCan I change my vote?
Ordinary Shares
If you hold ordinary shares of record and Warranties. Assubmit your proxy card to vote by mail, by email, or appoint a conditionproxy in advance of the Annual Meeting, you may change your vote by delivering a valid later-dated proxy within the time limitations set forth above, or voting at the Annual Meeting through the online meeting platform.
If you are a Non-Registered Holder of ordinary shares and vote through the electronic voting system of the Israeli Securities Authority, you may revoke your vote through such voting system at least six hours prior to the exercise of an option, the Committee may require the person exercising such option to represent and warrantAnnual Meeting (i.e., before 10:00 a.m., Israel time, on June 14, 2021), or by voting at the Annual Meeting through the online meeting platform. If you are a Non-Registered Holder of ordinary shares and submit your proxy card to vote by mail, by email, or appoint an authorized proxy in advance of the Annual Meeting, you may change your vote by delivering a valid later-dated proxy within the time limitations set forth above, or voting at the Annual Meeting through the online meeting platform and subject to the instructions set forth above.
Attendance at the Annual Meeting will not cause your previous vote to be revoked unless you specifically so request.
ADSs
If you are the record owner of any such exercise thatADSs, you must follow the instructions provided by the Depositary in order to change your vote. If you hold your ADSs are being purchased only for investment and without any present intentionthrough a broker, bank or other nominee, you must follow the instructions provided by your broker, bank or other nominee, in order to sell or distribute such ADSs if, inchange your vote. The last instructions you submit prior to the opinion of counsel fordeadline indicated by the CompanyDepositary or the Issuer, suchbroker, bank or other nominee, as applicable, will be used to instruct the Depositary how to vote the ordinary shares underlying your ADSs. Attendance at the Annual Meeting will not cause your previous vote to be revoked.
Additional information for holders of ordinary shares and ADSs on how to participate at the virtual Annual Meeting
| ∎ | Access the Annual Meeting online platform at www.meetingcenter.io/212475968 and enter the meeting password TEVA2021 and your 15-digit control number (for holders of ADSs) or Israeli identification number or company registration number (for holders of ordinary shares) beginning on Monday, June 14, 2021 at 3:45 p.m., Israel time, 8:45 a.m., Eastern time. |
| ∎ | To submit a question, visit www.meetingcenter.io/212475968 within 24 hours prior to or throughout the Annual Meeting with your control number and click on the messages icon to submit your question. |
| ∎ | If you encounter any technical difficulties with the meeting platform on the date of the Annual Meeting, please call the support team at the numbers listed on the log-in screen, technical support will be available during this time and will remain available until the Annual Meeting has ended. |
| ∎ | Whether or not you plan to attend the Annual Meeting, we urge you to vote and submit your proxy in advance by one of the methods described above and in the other proxy materials for the Annual Meeting. |
| ∎ | No recording of the Annual Meeting is allowed, including audio and video recording. |
Proxy Materials
Why did I receive a representation is required“Notice of Internet Availability of Proxy Materials” but no proxy materials?
We distribute our Notice of Annual Meeting of Shareholders, Proxy Statement and Annual Report (collectively, the “proxy materials”) to certain shareholders via the Internet under the “Notice and Access” approach permitted by anyrules of the aforementioned applicable provisionsSEC. This approach conserves natural resources and reduces our
| 94 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |
Questions and Answers about the Annual Meeting
distribution costs, while providing a timely and convenient method of law.accessing the materials and voting. On or by April 30, 2021, we expect to have mailed a “Notice of Internet Availability of Proxy Materials” to participating shareholders containing instructions on how to access the proxy materials on the Internet.
22. TermCan I access the proxy materials on the Internet?
The proxy materials are available on our website at www.tevapharm.com/2021proxymaterials. Information on our website is not part of Plan. The Plan was initially adoptedthe proxy materials and is not incorporated into the proxy statement by reference. Record owners of our ADSs may also access the proxy materials at www.investorvote.com/teva by following the instructions provided by the Issuer BoardDepositary. Beneficial owners of our ADSs may also access the proxy materials at www.proxyvote.com by following the instructions provided by their broker, bank or other nominee. Instead of receiving future proxy statements and accompanying materials by mail, most shareholders and ADS holders can elect to receive an e-mail that will provide electronic links to them. Opting to receive your proxy materials online will conserve natural resources and will save us the cost of producing documents and mailing them to you.The proxy materials are also available through Teva’s public filing on MAGNA (the Israeli Securities Authority’s electronic filing system) at www.magna.isa.gov.il, on the TASE’s website at www.maya.tase.co.il, or on the SEC’s website at www.sec.gov.
How do I request paper copies of the proxy materials at no charge?
You may contact Investor Relations by sending an email to TevaIR@tevapharm.com, or you may contact our proxy solicitor, MacKenzie Partners, Inc., by sending an email to proxy@mackenziepartners.com, by May 5, 2008,27, 2021.
If you are a record owner of ADSs, you may request proxy materials at www.investorvote.com/teva, by calling toll-free within the U.S. at (866) 641-4276 or by sending an email to investorvote@computershare.com, by May 27, 2021 and approvedfollowing the instructions provided by the shareholders of the Issuer on June 29, 2008, and was subsequently amended by the Issuer Board on February 4, 2016 to increase the Purchase Price from 85% to 95% of the Fair Market Value of an ADS on the applicable Exercise Date effective with the first Offering Period commencing on January 1, 2016. The Plan was subsequently amended and restated by the Issuer Board on September 7, 2017 (the “September 2017 Amendment”) to (a) increase the maximum numberDepositary.
If you are a beneficial owner of ADSs, authorized for issuance underyou may request proxy materials by following the Planinstructions at www.proxyvote.com or by calling toll free within the U.S. at (800) 579-1639 or by sending an additional 5,000,000 ADSs, (b) extendemail to sendmaterial@proxyvote.com by May 27, 2021 and following the terminstructions provided by your broker, bank or other nominee.
Other Questions
Could other matters be decided at the Annual Meeting?
The only items of business that our Board of Directors intends to present at the Plan for a term of ten years from the effective date of the September 2017 Amendment and (c) make certain other ministerial changes to the Plan. The Plan shall continue through September 7, 2027 if the September 2017 Amendment is approved by the shareholders of the Issuer and shall continue in effect through May 5, 2018 if the September 2017 Amendment is not approved by the shareholders of the Issuer, in each case, unless sooner terminated under Section 18 hereof.
23. Shareholder Approval. In the event that the September 2017 Amendment is not approved by the shareholders of the Issuer holding a majority of its outstanding ADSs and Ordinary Shares within twelve months of the September 2017 Amendment, the September 2017 Amendment shall automatically be null and void, and (a) the term of the Plan shall expire asAnnual Meeting are set forth in Section 22 above, (b) the maximum number of ADSs authorized for issuance under the Plan shall revert to 3,500,000 and (c) allthis Proxy Statement. As of the ADSs purchased in excessdate of this Proxy Statement, no shareholder has advised us of the 3,500,000 ADS maximum shallintent to present any other matter, and we are not aware of any other matter to be sold onpresented at the open marketAnnual Meeting. However, according and all payroll deductions for Plan participants in respect of such excess ADSs shall be returned to them, subject to the Israeli Companies Law and our Articles of Association, certain shareholders are entitled to propose items to the agenda. For more information, please see “Shareholder Proposals for the 2021 Annual Meeting and the 2022 Annual Meeting” above.
Who will pay for the cost of this proxy solicitation?
Teva will bear the entire cost of solicitation of proxies, including preparation, assembly, printing, and mailing of this Proxy Statement, the voting instruction card and any appropriate withholding.
* * *additional information furnished to shareholders. Teva may reimburse brokerage firms and other persons representing beneficial owners of ordinary shares or ADSs for reasonable expenses incurred by them in forwarding proxy soliciting materials to such beneficial owners. We retained MacKenzie Partners, Inc. to assist with the solicitation of proxies for a fee in the amount of $23,500, plus reimbursable expenses. In addition to solicitation by mail, certain of our directors, officers and regular employees, without additional remuneration, may solicit proxies by telephone, facsimile or personal contact.
| Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | 95 | |
Questions and Answers about the Annual Meeting
Who can I contact if I require further assistance?
If you need assistance in submitting your proxy or have questions regarding the Annual Meeting, please contact our Investor Relations department by email at TevaIR@tevapharm.com or by mail at Teva Pharmaceutical Industries Ltd., 124 Dvora HaNevi’a Street, Tel Aviv, 6944020 Israel, attention: Investor Relations, or by telephone at +972-3-914-8262. You may also contact our proxy solicitor, MacKenzie Partners, Inc., by email at proxy@mackenziepartners.com or by calling toll free within the U.S. at +1 (800) 322-2885 or outside the U.S. at + 1 (212) 929-5500.
| 96 | Teva Pharmaceutical Industries Ltd. 2021 Proxy Statement | |



TEVA PHARMACEUTICAL INDUSTRIES LIMITED (“TEVA”)
20182021 ANNUAL GENERAL MEETING OF SHAREHOLDERS TO BE HELD ON JUNE 5, 201814, 2021
PROXY CARD
THIS PROXY IS SOLICITED BY THE BOARD OF DIRECTORS OF TEVA
Teva’s Board of Directors recommends that you vote FOR Proposals 1, 2, 4 and 5 and that you vote ONE YEAR with respect to Proposal 3.all proposals. If you execute and return this proxy card without indicating any directions with respect to any matter, this proxy card will be voted FOR Proposals 1, 2, 4 and 5 and ONE YEAR with respect to Proposal 3, as applicable.all proposals.
Information in respect of the undersigned:
| Shareholder name: |
| |
Number of identity card or passport (country): |
| |
Corporation number: |
| |
Place of incorporation: |
| |
Number of Teva ordinary shares being voted: | ||
The undersigned hereby constitutes and appoints each of Messrs. SOL BARER, KÅRE SCHULTZDOV BERGWERK, DIKLA TADMOR and DOV BERGWERK,NETANEL DEROVAN, acting individually, the true and lawful attorney, agent and proxy of the undersigned, with full power of substitution, to vote with respect to the number of shares set forth above, standing in the name of the undersigned at the close of trading on the Record Date, at the 20182021 Annual General Meeting of Shareholders, and at any and all adjournments thereof, with all the power that the undersigned would possess if personally present and especially (but without limiting the general authorization and power hereby given) to vote as instructed on the reverse side.
In order to be counted, a duly executed proxy must be received by Teva by 4:3000 p.m., Israel time, on June 1, 201810, 2021 (if not revoked prior to such time), unless determined otherwise by the chairman of the meeting, by submitting this proxy card to Teva’s executive officesTeva at 5 Basel124 Dvora HaNevi’a Street, Petach Tikva, 4951033Tel Aviv, 6944020, Israel to the attention of the Corporate Secretary.Company Secretary or by email to TevaAGM2021@tevapharm.com.
In order to be counted, in addition to this proxy card,card: (i) shareholders registered in Teva’s shareholder register (Registered Holders) must also provide Teva with a copy of such Registered Holder’s identity card, passport or certificate of incorporation, as the case may be. Abe; and (ii) a shareholder registered pursuant to Section 177(1) of the Israeli Companies Law, 5759-1999, through a nominee company(Non-Registered Holders) must also provide Teva with an ownership certificate confirming suchNon-Registered Holder’s ownership of Teva’s ordinary shares on the Record Date, which certificate must be approved by a member of the Tel Aviv Stock Exchange, as required by the Israeli Companies Regulations (Proof of Share Ownership for Voting at a General Meeting), 5760-2000.Non-Registered Holders may alternatively submit their votes through the electronic voting system of the Israeli Securities Authority athttps//:votes.isa.gov.il.
This proxy card, when properly executed, will be voted in the manner directed herein by the undersigned. Any and all proxies heretofore given are hereby revoked.
(Continued and to be signed on the reverse side)
PLEASE COMPLETE, SIGN, DATE AND RETURN PROMPTLY
Matter on the Agenda: | Please vote by marking “X” in the | |||||||||||||
For | Against | Abstain | ||||||||||||
| 1. |
ELECTION OF DIRECTORS: | |||||||||||||
(a) Rosemary A. Crane
| ||||||||||||||
(b)
| ||||||||||||||
(c) Gerald M. Lieberman | ||||||||||||||
(d) Prof. Ronit Satchi-Fainaro
| ||||||||||||||
| 2. |
| |||||||||||||
| 3. |
|
|
|
|
| |||||||||
|
|
| ||||||||||||
| ||||||||||||||
AS TEVA’S INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM UNTIL TEVA’S 2022 ANNUAL MEETING OF SHAREHOLDERS | ||||||||||||||
| Signature | Date |